Dynamic interactions of excitatory and inhibitory inputs in hypoglossal motoneurones: respiratory phasing and modulation by PKA
- PMID: 14660708
- PMCID: PMC1664783
- DOI: 10.1113/jphysiol.2003.054528
Dynamic interactions of excitatory and inhibitory inputs in hypoglossal motoneurones: respiratory phasing and modulation by PKA
Abstract
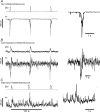
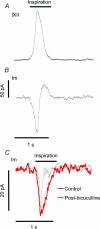
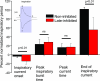
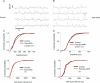
The balance of excitation and inhibition converging upon a neurone is a principal determinant of neuronal output. We investigated the role of inhibition in shaping and gating inspiratory drive to hypoglossal (XII) motoneuronal activity. In neonatal rat medullary slices that generate a spontaneous respiratory rhythm, patch-clamp recordings were made from XII motoneurones, which were divided into three populations according to their inhibitory inputs: non-inhibited, inspiratory-inhibited and late-inspiratory-inhibited. In late-inspiratory-inhibited motoneurones, blockade of GABA(A) receptors with bicuculline abolished inspiratory-phased inhibition and increased the duration of inspiratory drive currents. In inspiratory-inhibited motoneurones, bicuculline abolished phasic inhibition, which frequently revealed excitatory inspiratory drive currents. In non-inhibited motoneurones, neither bicuculline nor strychnine markedly changed inspiratory drive currents. Inhibitory currents in XII motoneurones were potentiated by protein kinase A (PKA) activity. Intracellular dialysis of the catalytic subunit of PKA or bath application of the PKA activator Sp-cAMP significantly increased the amplitude of expiratory-phased IPSCs without any change in IPSP frequency. Inspiratory-phased inhibition in inspiratory-inhibited motoneurones was potentiated by Sp-cAMP. We conclude that inspiratory-phased inhibition is prevalent in neonatal XII motoneurones and plays an important role in shaping motoneuronal output. These inhibitory inputs are modulated by PKA, which also modulates excitatory inputs.
Figures



Similar articles
-
Protein kinase G-dependent mechanisms modulate hypoglossal motoneuronal excitability and long-term facilitation.J Physiol. 2010 Nov 15;588(Pt 22):4431-9. doi: 10.1113/jphysiol.2010.194209. Epub 2010 Sep 20. J Physiol. 2010. PMID: 20855434 Free PMC article.
-
Modulation of AMPA receptors by cAMP-dependent protein kinase in preBötzinger complex inspiratory neurons regulates respiratory rhythm in the rat.J Physiol. 2003 Mar 1;547(Pt 2):543-53. doi: 10.1113/jphysiol.2002.031005. Epub 2003 Jan 24. J Physiol. 2003. PMID: 12562968 Free PMC article.
-
Dynamic modulation of inspiratory drive currents by protein kinase A and protein phosphatases in functionally active motoneurons.J Neurosci. 2003 Feb 15;23(4):1099-103. doi: 10.1523/JNEUROSCI.23-04-01099.2003. J Neurosci. 2003. PMID: 12598595 Free PMC article.
-
Respiratory rhythm generation and synaptic inhibition of expiratory neurons in pre-Bötzinger complex: differential roles of glycinergic and GABAergic neural transmission.J Neurophysiol. 1997 Apr;77(4):1853-60. doi: 10.1152/jn.1997.77.4.1853. J Neurophysiol. 1997. PMID: 9114241
-
Modulation of hypoglossal motoneuron excitability by intracellular signal transduction cascades.Respir Physiol Neurobiol. 2005 Jul 28;147(2-3):131-43. doi: 10.1016/j.resp.2005.03.014. Respir Physiol Neurobiol. 2005. PMID: 15893504 Review.
Cited by
-
Cyclothiazide-induced persistent increase in respiratory-related activity in vitro.J Physiol. 2012 Oct 1;590(19):4897-915. doi: 10.1113/jphysiol.2012.232421. Epub 2012 Jul 2. J Physiol. 2012. PMID: 22753547 Free PMC article.
-
Multiple phases of excitation and inhibition in central respiratory drive potentials of thoracic motoneurones in the rat.J Physiol. 2010 Aug 1;588(Pt 15):2731-44. doi: 10.1113/jphysiol.2009.186346. Epub 2010 Jun 2. J Physiol. 2010. PMID: 20519317 Free PMC article.
-
Protein kinase G-dependent mechanisms modulate hypoglossal motoneuronal excitability and long-term facilitation.J Physiol. 2010 Nov 15;588(Pt 22):4431-9. doi: 10.1113/jphysiol.2010.194209. Epub 2010 Sep 20. J Physiol. 2010. PMID: 20855434 Free PMC article.
-
Divisive gain modulation of motoneurons by inhibition optimizes muscular control.J Neurosci. 2015 Feb 25;35(8):3711-23. doi: 10.1523/JNEUROSCI.3899-14.2015. J Neurosci. 2015. PMID: 25716868 Free PMC article.
-
Spinal activation of the cAMP-PKA pathway induces respiratory motor recovery following high cervical spinal cord injury.Brain Res. 2008 Sep 26;1232:206-13. doi: 10.1016/j.brainres.2008.07.012. Epub 2008 Jul 12. Brain Res. 2008. PMID: 18656458 Free PMC article.
References
-
- Cohen M, Feldman JL. Discharge properties of dorsal medullary neurons: Relation to pulmonary afferent and phrenic efferent discharges. J Neurophysiol. 1984;4:753–776. - PubMed
-
- Cohen M, Huang W-X, Barnhardt R, See W. Timing of medullary late-inspiratory neuron discharges: vagal afferent effects indicate possible off-swich function. J Neurophysiol. 1993;69:1784–1787. - PubMed
-
- DeVente J, Asan E, Gambaryan S, Ittersum MM-V, Axter H, Gallatz K, Lohmann S, Palkovitz M. Localization of cGMP-dependant protein kinase type II in rat brain. Neurosci. 2001;108:27–49. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

