Mechanisms for pituitary tumorigenesis: the plastic pituitary
- PMID: 14660734
- PMCID: PMC281651
- DOI: 10.1172/JCI20401
Mechanisms for pituitary tumorigenesis: the plastic pituitary
Abstract
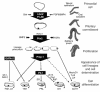
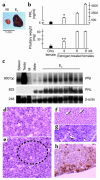
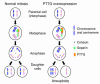
The anterior pituitary gland integrates the repertoire of hormonal signals controlling thyroid, adrenal, reproductive, and growth functions. The gland responds to complex central and peripheral signals by trophic hormone secretion and by undergoing reversible plastic changes in cell growth leading to hyperplasia, involution, or benign adenomas arising from functional pituitary cells. Discussed herein are the mechanisms underlying hereditary pituitary hypoplasia, reversible pituitary hyperplasia, excess hormone production, and tumor initiation and promotion associated with normal and abnormal pituitary differentiation in health and disease.
Figures
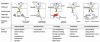
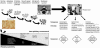

References
-
- Taniguchi Y, Yasutaka S, Kominami R, Shinohara H. Proliferation and differentiation of rat anterior pituitary cells. Anat. Embryol. (Berl.). 2002; 206:1–11. - PubMed
-
- Levy A, Lightman S. Molecular defects in the pathogenesis of pituitary tumours. Front. Neuroendocrinol. 2003; 24:94–127. - PubMed
-
- Asa SL, Ezzat S. The cytogenesis and pathogenesis of pituitary adenomas. Endocr. Rev. 1998; 19:798–827. - PubMed
-
- Faglia G, Spada A. Genesis of pituitary adenomas: state of the art. J. Neurooncol. 2001; 54:95–110. - PubMed
-
- Prezant TR, Melmed S. Molecular pathogenesis of pituitary disorders. Current Opinion in Endocrinology & Diabetes. 2002; 9:61–78.

