Proinflammatory functions of vascular endothelial growth factor in alloimmunity
- PMID: 14660742
- PMCID: PMC281640
- DOI: 10.1172/JCI17712
Proinflammatory functions of vascular endothelial growth factor in alloimmunity
Abstract
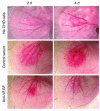
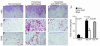
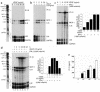
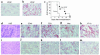
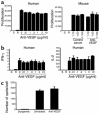
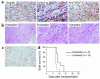
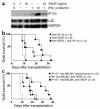
Vascular endothelial growth factor (VEGF), an established angiogenesis factor, is expressed in allografts undergoing rejection, but its function in the rejection process has not been defined. Here, we initially determined that VEGF is functional in the trafficking of human T cells into skin allografts in vivo in the humanized SCID mouse. In vitro, we found that VEGF enhanced endothelial cell expression of the chemokines monocyte chemoattractant protein 1 and IL-8, and in combination with IFN-gamma synergistically induced endothelial cell production of the potent T cell chemoattractant IFN-inducible protein-10 (IP-10). Treatment of BALB/c (H-2d) recipients of fully MHC-mismatched C57BL/6 (H-2b) donor hearts with anti-VEGF markedly inhibited T cell infiltration of allografts and acute rejection. Anti-VEGF failed to inhibit T cell activation responses in vivo, but inhibited intragraft expression of several endothelial cell adhesion molecules and chemokines, including IP-10. In addition, whereas VEGF expression was increased, neovascularization was not associated with acute rejection, and treatment of allograft recipients with the angiogenesis inhibitor endostatin failed to inhibit leukocyte infiltration of the grafts. Thus, VEGF appears to be functional in acute allograft rejection via its effects on leukocyte trafficking. Together, these observations provide mechanistic insight into the proinflammatory function of VEGF in immunity.
Figures


References
-
- Auerbach R, Sidky YA. Nature of the stimulus leading to lymphocyte-induced angiogenesis. J. Immunol. 1979; 123:751–754. - PubMed
-
- Cotran, R.S. 1994. Inflammation and repair. In Pathologic basis of disease. R.S. Cotran, V. Kumar, and S.L. Robbins, editors. WB Saunders, Philadelphia, USA. 51–92.
-
- Moulton KS, et al. Angiogenesis in the huPBL-SCID model of human transplant rejection. Transplantation. 1999; 67:1626–1631. - PubMed
-
- Leung DW, Cachianes G, Kuang WJ, Goeddel DV, Ferrara N. Vascular endothelial growth factor is a secreted angiogenic mitogen. Science. 1989; 246:1306–1309. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

