A congenital mutation of the novel gene LRRC8 causes agammaglobulinemia in humans
- PMID: 14660746
- PMCID: PMC281644
- DOI: 10.1172/JCI18937
A congenital mutation of the novel gene LRRC8 causes agammaglobulinemia in humans
Abstract
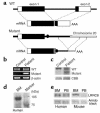
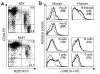
A girl with congenital agammaglobulinemia and minor facial anomalies lacked B cells in peripheral blood: karyotypic analysis of white blood cells showed balanced translocation, t(9;20)(q33.2;q12). In the current study, we isolated a novel gene, leucine-rich repeat-containing 8 (LRRC8), at the translocation site on chromosome 9. It has four transmembrane helices with one isolated and eight sequentially located leucine-rich repeats (LRRs) and constitutes a new protein family. It is expressed on T cells as well as on B-lineage cells. Translocation truncates the LRRC8 gene, resulting in deletion of the eighth, ninth, and half of the seventh LRR domains located close to the C-terminal. The truncated form of the LRRC8 gene is transcribed with sequences from the noncoding region adjacent to the truncated seventh LRR. Protein products derived from the truncated gene are coexpressed on white blood cells with the intact LRRC8 protein from the untranslocated allele. Transplantation experiments with murine bone marrow cells that were forced to express the truncated LRRC8 show that expression of the truncated protein inhibited B cell development. These results indicate that LRRC8 is responsible for the B cell deficiency in this patient and is required for B cell development.
Figures



Comment in
-
Genes required for B cell development.J Clin Invest. 2003 Dec;112(11):1636-8. doi: 10.1172/JCI20408. J Clin Invest. 2003. PMID: 14660738 Free PMC article.
References
-
- Urbanek P, Wang ZQ, Fetka I, Wagner EF, Busslinger M. Complete block of early B cell differentiation and altered patterning of the posterior midbrain in mice lacking Pax5/BSAP. Cell. 1994; 79:901–912. - PubMed
-
- Nutt SL, Heavey B, Rolink AG, Busslinger M. Commitment to the B-lymphoid lineage depends on the transcription factor Pax5. Nature. 1999; 401:556–562. - PubMed
-
- Schaniel C, Gottar M, Roosnek E, Melchers F, Rolink AG. Extensive in vivo self-renewal, long-term reconstitution capacity, and hematopoietic multipotency of Pax5-deficient precursor B-cell clones. Blood. 2002; 99:2760–2766. - PubMed
-
- Lin H, Grosschedl R. Failure of B-cell differentiation in mice lacking the transcription factor EBF. Nature. 1995; 376:263–267. - PubMed
-
- Zhuang Y, Soriano P, Weintraub H. The helix-loop-helix gene E2A is required for B cell formation. Cell. 1994; 79:875–884. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

