Myc confers androgen-independent prostate cancer cell growth
- PMID: 14660748
- PMCID: PMC281646
- DOI: 10.1172/JCI19035
Myc confers androgen-independent prostate cancer cell growth
Abstract
Prostate cancer is one of the most diagnosed and mortal cancers in western countries. A major clinical problem is the development of androgen-independent prostate cancer (AIPC) during antihormonal treatment. The molecular mechanisms underlying the change from androgen dependence to independence of these tumors are poorly understood and represent a challenge to develop new therapies. Based on genetic data showing amplification of the c-myc gene in AIPC, we studied the ability of c-myc to confer AIPC cell growth. Human androgen-dependent prostate cancer cells overexpressing c-myc grew independently of androgens and presented tumorigenic properties in androgen-depleted conditions. Analysis of signalling pathways by pharmacological inhibitors of the androgen receptor (AR) or by RNA interference directed against AR or c-myc showed that c-myc acted downstream of AR through multiple growth effectors. Thus c-myc is required for androgen-dependent growth and following ectopic expression can induce androgen-independent growth. Moreover, RNA interference directed against c-myc showed that growth of human AIPC cells, AR-positive or -negative, required c-myc expression. Furthermore, we showed that c-myc-overexpressing cells retain a functional p53 pathway and thus respond to etoposide.
Figures

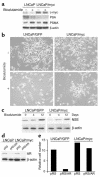
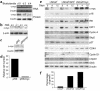
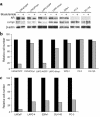
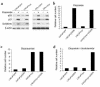
References
-
- Feldman BJ, Feldman D. The development of androgen-independent prostate cancer. Nat. Rev. Cancer. 2001; 1:34–45. - PubMed
-
- Craft N, Shostak Y, Carey M, Sawyers CL. A mechanism for hormone-independent prostate cancer through modulation of androgen receptor signaling by the HER-2/neu tyrosine kinase. Nat. Med. 1999; 5:280–285. - PubMed
-
- Taplin ME, et al. Selection for androgen receptor mutations in prostate cancers treated with androgen antagonist. Cancer Res. 1999; 59:2511–2515. - PubMed
-
- Zhao XY, et al. Glucocorticoids can promote androgen-independent growth of prostate cancer cells through a mutated androgen receptor. Nat. Med. 2000; 6:703–706. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

