The tetraspanin CD63 enhances the internalization of the H,K-ATPase beta-subunit
- PMID: 14660791
- PMCID: PMC307607
- DOI: 10.1073/pnas.2536699100
The tetraspanin CD63 enhances the internalization of the H,K-ATPase beta-subunit
Abstract
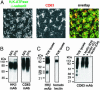
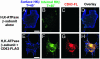
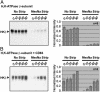
The tetraspanin CD63 resides in late endosomes, lysosomes, secretory vesicles, and at the plasma membrane, and it moves among these compartments. We find that CD63 is present also in tubulovesicular elements, the intracellular compartments that contain the H,K-ATPase in unstimulated gastric parietal cells. The H,K-ATPase beta-subunit and CD63 colocalize in parietal cells and form a complex that can be coprecipitated. The beta-subunit and CD63 also interact when they are coexpressed in COS-7 cells. Furthermore, expression with CD63 induces the redistribution of the beta-subunit from the cell surface to CD63+ intracellular compartments. Immunofluorescence and biochemical experiments reveal that this redistribution occurs by enhanced endocytosis of H,K-ATPase beta-subunit complexed with CD63. Coexpression of the beta-subunit with mutant CD63 polypeptides demonstrates that the enhanced internalization of the beta-subunit depends on the capacity of CD63 to interact with adaptor protein complexes 2 and 3. These data indicate that CD63 serves as an adaptor protein that links its interaction partners to the endocytic machinery of the cell and suggest a previously uncharacterized protein-trafficking role for the tetraspanins.
Figures




References
-
- Maecker, H. T., Todd, S. C. & Levy, S. (1997) FASEB J. 11, 428–442. - PubMed
-
- Sincock, P. M., Mayrhofer, G. & Ashman, L. K. (1997) J. Histochem. Cytochem. 45, 515–525. - PubMed
-
- Escola, J. M., Kleijmeer, M. J., Stoorvogel, W., Griffith, J. M., Yoshie, O. & Geuze, H. J. (1998) J. Biol. Chem. 273, 20121–20127. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

