Plastid protein synthesis is required for plant development in tobacco
- PMID: 14660796
- PMCID: PMC307636
- DOI: 10.1073/pnas.2533668100
Plastid protein synthesis is required for plant development in tobacco
Abstract
Chloroplasts fulfill important functions in cellular metabolism. The majority of plastid genome-encoded genes is involved in either photosynthesis or chloroplast gene expression. Whether or not plastid genes also can determine extraplastidic functions has remained controversial. We demonstrate here an essential role of plastid protein synthesis in tobacco leaf development. By using chloroplast transformation, we have developed an experimental system that produces recombination-based knockouts of chloroplast translation in a cell-line-specific manner. The resulting plants are chimeric and, in the presence of translational inhibitors, exhibit severe developmental abnormalities. In the absence of active plastid protein synthesis, leaf blade development is abolished because of an apparent arrest of cell division. This effect appears to be cell-autonomous in that adjacent sectors of cells with translating plastids are phenotypically normal but cannot complement for the absence of plastid translation in mutant sectors. Developmental abnormalities also are seen in flower morphology, indicating that the defects are not caused by inhibited expression of plastid photosynthesis genes. Taken together, our data point to an unexpected essential role of plastid genes and gene expression in plant development and cell division.
Figures



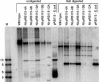
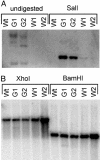
References
-
- Kaneko, T., Sato, S., Kotani, H., Tanaka, A., Asamizu, E., Nakamura, Y., Miyajima, N., Hirosawa, M., Sugiura, M., Sasamoto, S., et al. (1996) DNA Res. 3, 109–136. - PubMed
-
- Kaneko, T. & Tabata, S. (1997) Plant Cell Physiol. 38, 1171–1176. - PubMed
-
- Kotani, H. & Tabata, S. (1998) Annu. Rev. Plant Physiol. Plant Mol. Biol. 49, 151–171. - PubMed
-
- Wakasugi, T., Tsudzuki, T. & Sugiura, M. (2001) Photosynthesis Res. 70, 107–118. - PubMed
-
- Jarvis, P. (2001) Curr. Biol. 11, 307–310. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

