Primary ovarian cancer chemotherapy: current standards of care
- PMID: 14661040
- PMCID: PMC2750616
- DOI: 10.1038/sj.bjc.6601494
Primary ovarian cancer chemotherapy: current standards of care
Abstract
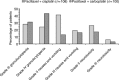
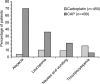
Chemotherapy has been regarded as standard therapy for the majority of women with advanced epithelial ovarian cancer for several decades, with this role filled largely by the alkylating agents - used as monotherapy - until the mid-1980s. The activity of cisplatin in this disorder was established during the 1970s, and combinations of cisplatin and an alkylating agent were widely used during the late 1980s. However, further research prompted by continuing concerns over poor survival and tolerability led to the adoption of paclitaxel in combination with either cisplatin or carboplatin as first-line therapy in ovarian cancer during the 1990s. Most recent research has focused on further optimisation of these regimens to maximise clinical benefit while minimising toxicity, and investigations into alternative taxanes (e.g. docetaxel), other novel agents and new treatment schedules are ongoing.
Figures

References
-
- Aabo K, Adams M, Adnitt P, Alberts DS, Barley V, Bell DR, Bianchi U, Bolis G, Brady MF, Brodovsky HS, Bruckner H, Buyse M, Canetta R, Chylak V, Cohen CJ, Colombo N, Conte PF, Crowther D, Edmonson JH, Gennatas C, Gilbey E, Gore M, Guthrie D, Yeap BY (1998) Chemotherapy in advanced ovarian cancer: four systematic meta-analyses of individual patient data from 37 randomized trials. Advanced Ovarian Cancer Trialists' Group. Br J Cancer 78: 1479–1487 - PMC - PubMed
-
- Advanced Ovarian Cancer Trialists' Group (2000) Chemotherapy for advanced ovarian cancer. Cochrane Database Syst Rev 2: CD001418 - PubMed
-
- A'Hern RP, Gore ME (1995) Impact of doxorubicin on survival in advanced ovarian cancer. J Clin Oncol 13: 726–732 - PubMed
-
- American Cancer Society (2003) What are the key statistics about ovarian cancer? Available from: URL: http://www.cancer.org/docroot/CRI/content/CRI_2_4_1X_What_are_the_key_st... 6 July 2003]
-
- Beers MH, Berkow R (eds) 1999) Ovarian cancer. In The Merck Manual of Diagnosis and Therapy, 17th edn, pp 1962–1964. Whitehouse Station, NJ: Merck Research Laboratories
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

