Interleukin 7 regulates the survival and generation of memory CD4 cells
- PMID: 14662907
- PMCID: PMC2194153
- DOI: 10.1084/jem.20030735
Interleukin 7 regulates the survival and generation of memory CD4 cells
Abstract
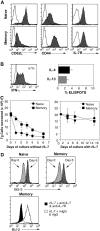
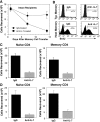
Cytokines, particularly those of the common gamma chain receptor family, provide extrinsic signals that regulate naive CD4 cell survival. Whether these cytokines are required for the maintenance of memory CD4 cells has not been rigorously assessed. In this paper, we examined the contribution of interleukin (IL) 7, a constitutively produced common gamma chain receptor cytokine, to the survival of resting T cell receptor transgenic memory CD4 cells that were generated in vivo. IL-7 mediated the survival and up-regulation of Bcl-2 by resting memory CD4 cells in vitro in the absence of proliferation. Memory CD4 cells persisted for extended periods upon adoptive transfer into intact or lymphopenic recipients, but not in IL-7- mice or in recipients that were rendered deficient in IL-7 by antibody blocking. Both central (CD62L+) and effector (CD62L-) memory phenotype CD4 cells required IL-7 for survival and, in vivo, memory cells were comparable to naive CD4 cells in this regard. Although the generation of primary effector cells from naive CD4 cells and their dissemination to nonlymphoid tissues were not affected by IL-7 deficiency, memory cells failed to subsequently develop in either the lymphoid or nonlymphoid compartments. The results demonstrate that IL-7 can have previously unrecognized roles in the maintenance of memory in the CD4 cell population and in the survival of CD4 cells with a capacity to become memory cells.
Figures


References
-
- Welsh, R.M., and L.K. Selin. 2002. No one is naive: the significance of heterologous T-cell immunity. Nat. Rev. Immunol. 2:417–426. - PubMed
-
- Homann, D., L. Teyton, and M.B.A. Oldstone. 2001. Differential regulation of antiviral T-cell immunity results in stable CD8+ but declining CD4+ T cell memory. Nat. Med. 7:913–919. - PubMed
-
- Varga, S.M., L.K. Selin, and R.M. Welsh. 2001. Independent regulation of lymphocytic choriomeningitis virus-specific T cell memory pools: relative stability of CD4 memory under conditions of CD8 memory T cell loss. J. Immunol. 166:1554–1561. - PubMed
-
- Ernst, B., D.-S. Lee, J.M. Chang, J. Sprent, and C.D. Surh. 1999. The peptide ligands mediating positive selection in the thymus control T cell survival and homeostatic proliferation in the periphery. Immunity. 11:173–181. - PubMed
-
- Tanchot, C.F., A. Lemonnier, B. Perarnau, and A.A. Freitas. 1997. Differential requirements for survival and proliferation of CD8 naive or memory T cells. Science. 276:2057–2062. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

