Loop-mediated isothermal amplification for detection of African trypanosomes
- PMID: 14662933
- PMCID: PMC308967
- DOI: 10.1128/JCM.41.12.5517-5524.2003
Loop-mediated isothermal amplification for detection of African trypanosomes
Abstract
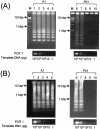
While PCR is a method of choice for the detection of African trypanosomes in both humans and animals, the expense of this method negates its use as a diagnostic method for the detection of endemic trypanosomiasis in African countries. The loop-mediated isothermal amplification (LAMP) reaction is a method that amplifies DNA with high specificity, efficiency, and rapidity under isothermal conditions with only simple incubators. An added advantage of LAMP over PCR-based methods is that DNA amplification can be monitored spectrophotometrically and/or with the naked eye without the use of dyes. Here we report our conditions for a highly sensitive, specific, and easy diagnostic assay based on LAMP technology for the detection of parasites in the Trypanosoma brucei group (including T. brucei brucei, T. brucei gambiense, T. brucei rhodesiense, and T. evansi) and T. congolense. We show that the sensitivity of the LAMP-based method for detection of trypanosomes in vitro is up to 100 times higher than that of PCR-based methods. In vivo studies in mice infected with human-infective T. brucei gambiense further highlight the potential clinical importance of LAMP as a diagnostic tool for the identification of African trypanosomiasis.
Figures



References
-
- Akane, A., K. Matsubara, H. Nakamura, S. Takahashi, and K. Kimura. 1994. Identification of the heme compound copurified with deoxyribonucleic acid (DNA) from bloodstains, a major inhibitor of polymerase chain reaction (PCR) amplification. J. Forensic Sci. 39:362-372. - PubMed
-
- Artama, W. T., M. W. Agey, and J. E. Donelson. 1992. DNA comparisons of Trypanosoma evansi (Indonesia) and Trypanosoma brucei spp. Parasitology 104:67-74. - PubMed
-
- Avarzed, A., D. T. De Waal, I. Igarashi, A. Sato, T. Oyamada, Y. Toyoda, and N. Suzuki. 1997. Prevalence of equine piroplasmosis in Central Mongolia. Onderstepoort J. Vet. Res. 64:141-145. - PubMed
-
- Belec, L., J. Authier, M. C. Eliezer-Vanerot, C. Piedouillet, A. S. Mohamed, and R. K. Gherardi. 1998. Myoglobin as a polymerase chain reaction (PCR) inhibitor: a limitation for PCR from skeletal muscle tissue avoided by the use of Thermus thermophilus polymerase. Muscle Nerve 21:1064-1067. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

