Hypervirulent mutant of Mycobacterium tuberculosis resulting from disruption of the mce1 operon
- PMID: 14663145
- PMCID: PMC307668
- DOI: 10.1073/pnas.2433882100
Hypervirulent mutant of Mycobacterium tuberculosis resulting from disruption of the mce1 operon
Abstract
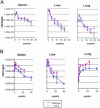
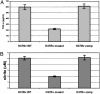
An estimated one-third of the world's population is latently infected with Mycobacterium tuberculosis, the etiologic agent of tuberculosis. Here, we demonstrate that, unlike wild-type M. tuberculosis, a strain of M. tuberculosis disrupted in the mce1 operon was unable to enter a stable persistent state of infection in mouse lungs. Instead, the mutant continued to replicate and killed the mice more rapidly than did the wild-type strain. Histological examination of mouse lungs infected with the mutant strain revealed diffusely organized granulomas with aberrant inflammatory cell migration. Murine macrophages infected ex vivo with the mutant strain were reduced in their ability to produce tumor necrosis factor alpha, IL-6, monocyte chemoattractant protein 1, and nitric oxide (NO), but not IL-4. The mce1 mutant strain complemented with the mce1 genes stimulated tumor necrosis factor alpha and NO production by murine macrophages at levels stimulated by the wild-type strain. These observations indicate that the mce1 operon mutant is unable to stimulate T helper 1-type immunity in mice. The hypervirulence of the mutant strain may have resulted from its inability to stimulate a proinflammatory response that would otherwise induce organized granuloma formation and control the infection without killing the organism. The mce1 operon of M. tuberculosis may be involved in modulating the host inflammatory response in such a way that the bacterium can enter a persistent state without being eliminated or causing disease in the host.
Figures




References
-
- Parrish, N. M., Dick, J. D. & Bishai, W. R. (1998) Trends Microbiol. 6, 107-112. - PubMed
-
- Mckinney, J. D., zu Bentrup, D. H., Munoz-Elias, E. J., Miczak, A. Cehn, B., Chan, W., Swenson, D., Sacchettini, J. C., Jacobs, W. R. & Russell, D. G. (2000) Nature 406, 735-738. - PubMed
-
- Glickman, M. S., Cox, J. S. & Jacobs, W. R. (2000) Mol. Cell 5, 717-727. - PubMed
-
- Arruda, S., Bomfim, G., Knights, R., Huima-Byron, T. & Riley, L. W. (1993) Science 261, 1454-1457. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

