Marker succession during the development of keratinocytes from cultured human embryonic stem cells
- PMID: 14663151
- PMCID: PMC307618
- DOI: 10.1073/pnas.0307226100
Marker succession during the development of keratinocytes from cultured human embryonic stem cells
Abstract
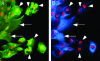
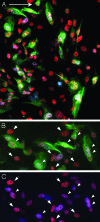
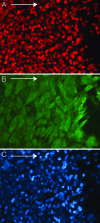
Human embryonic stem cells injected into scid mice produce nodules containing differentiated somatic tissues. From the trypsinized cells of such a nodule, we have recovered keratinocytes that can be grown in cell culture. The method of recovery is sensitive enough to detect small numbers of keratinocytes formed in the nodule, but for purposes of analysis, it is preferable to study the development of the entire keratinocyte lineage in culture. The principle of our analysis is the successive appearance of markers, including transcription factors with considerable specificity for the keratinocyte (p63 and basonuclin) and differentiation markers characteristic of its final state (keratin 14 and involucrin). We have determined the order of marker succession during the time- and migration-dependent development of keratinocytes from single embryoid bodies in cell culture. Of the markers we have examined, p63 was the earliest to appear in the keratinocyte lineage. The successive accumulation of later markers provides increasing certainty of emergence of the definitive keratinocyte.
Figures



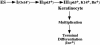
References
-
- Evans, M. J. & Kaufman, M. H. (1981) Nature 292, 154–156. - PubMed
-
- Smith, A. G. (2001) Annu. Rev. Cell Dev. Biol. 17, 435–462. - PubMed
-
- Bagutti, C., Wobus, A. M., Fassler, R. & Watt, F. M. (1996) Dev. Biol. 179, 184–196. - PubMed
-
- Bagutti, C., Hutter, C., Chiquet-Ehrismann, R., Fassler, R. & Watt, F. M. (2001) Dev. Biol. 231, 321–333. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

