Quorum sensing in nitrogen-fixing rhizobia
- PMID: 14665677
- PMCID: PMC309046
- DOI: 10.1128/MMBR.67.4.574-592.2003
Quorum sensing in nitrogen-fixing rhizobia
Abstract
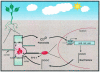
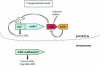
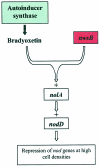
Members of the rhizobia are distinguished for their ability to establish a nitrogen-fixing symbiosis with leguminous plants. While many details of this relationship remain a mystery, much effort has gone into elucidating the mechanisms governing bacterium-host recognition and the events leading to symbiosis. Several signal molecules, including plant-produced flavonoids and bacterially produced nodulation factors and exopolysaccharides, are known to function in the molecular conversation between the host and the symbiont. Work by several laboratories has shown that an additional mode of regulation, quorum sensing, intercedes in the signal exchange process and perhaps plays a major role in preparing and coordinating the nitrogen-fixing rhizobia during the establishment of the symbiosis. Rhizobium leguminosarum, for example, carries a multitiered quorum-sensing system that represents one of the most complex regulatory networks identified for this form of gene regulation. This review focuses on the recent stream of information regarding quorum sensing in the nitrogen-fixing rhizobia. Seminal work on the quorum-sensing systems of R. leguminosarum bv. viciae, R. etli, Rhizobium sp. strain NGR234, Sinorhizobium meliloti, and Bradyrhizobium japonicum is presented and discussed. The latest work shows that quorum sensing can be linked to various symbiotic phenomena including nodulation efficiency, symbiosome development, exopolysaccharide production, and nitrogen fixation, all of which are important for the establishment of a successful symbiosis. Many questions remain to be answered, but the knowledge obtained so far provides a firm foundation for future studies on the role of quorum-sensing mediated gene regulation in host-bacterium interactions.
Figures





Similar articles
-
Environmental signals and regulatory pathways that influence exopolysaccharide production in rhizobia.Int J Mol Sci. 2011;12(11):7898-933. doi: 10.3390/ijms12117898. Epub 2011 Nov 15. Int J Mol Sci. 2011. PMID: 22174640 Free PMC article. Review.
-
An Alkane Sulfonate Monooxygenase Is Required for Symbiotic Nitrogen Fixation by Bradyrhizobium diazoefficiens (syn. Bradyrhizobium japonicum) USDA110T.Appl Environ Microbiol. 2019 Nov 27;85(24):e01552-19. doi: 10.1128/AEM.01552-19. Print 2019 Dec 15. Appl Environ Microbiol. 2019. PMID: 31562172 Free PMC article.
-
Nonnodulating Bradyrhizobium spp. Modulate the Benefits of Legume-Rhizobium Mutualism.Appl Environ Microbiol. 2016 Aug 15;82(17):5259-68. doi: 10.1128/AEM.01116-16. Print 2016 Sep 1. Appl Environ Microbiol. 2016. PMID: 27316960 Free PMC article.
-
Sinorhizobium medicae WSM419 Genes That Improve Symbiosis between Sinorhizobium meliloti Rm1021 and Medicago truncatula Jemalong A17 and in Other Symbiosis Systems.Appl Environ Microbiol. 2021 Jul 13;87(15):e0300420. doi: 10.1128/AEM.03004-20. Epub 2021 Jul 13. Appl Environ Microbiol. 2021. PMID: 33990306 Free PMC article.
-
What determines symbiotic nitrogen fixation efficiency in rhizobium: recent insights into Rhizobium leguminosarum.Arch Microbiol. 2023 Aug 5;205(9):300. doi: 10.1007/s00203-023-03640-7. Arch Microbiol. 2023. PMID: 37542687 Review.
Cited by
-
Environmental factors affecting the expression of pilAB as well as the proteome and transcriptome of the grass endophyte Azoarcus sp. strain BH72.PLoS One. 2012;7(1):e30421. doi: 10.1371/journal.pone.0030421. Epub 2012 Jan 20. PLoS One. 2012. PMID: 22276194 Free PMC article.
-
Environmental signals and regulatory pathways that influence exopolysaccharide production in rhizobia.Int J Mol Sci. 2011;12(11):7898-933. doi: 10.3390/ijms12117898. Epub 2011 Nov 15. Int J Mol Sci. 2011. PMID: 22174640 Free PMC article. Review.
-
Deciphering the Symbiotic Significance of Quorum Sensing Systems of Sinorhizobium fredii HH103.Microorganisms. 2020 Jan 2;8(1):68. doi: 10.3390/microorganisms8010068. Microorganisms. 2020. PMID: 31906451 Free PMC article.
-
Host-Associated Rhizobial Fitness: Dependence on Nitrogen, Density, Community Complexity, and Legume Genotype.Appl Environ Microbiol. 2022 Aug 9;88(15):e0052622. doi: 10.1128/aem.00526-22. Epub 2022 Jul 19. Appl Environ Microbiol. 2022. PMID: 35852362 Free PMC article.
-
Quorum size of Pseudomonas syringae is small and dictated by water availability on the leaf surface.Proc Natl Acad Sci U S A. 2008 Feb 26;105(8):3082-7. doi: 10.1073/pnas.0711723105. Epub 2008 Feb 19. Proc Natl Acad Sci U S A. 2008. PMID: 18287070 Free PMC article.
References
-
- Alt-Morbe, J., J. L. Stryker, C. Fuqua, P. L. Li, S. K. Farrand, and S. C. Winans. 1996. The conjugal transfer system of Agrobacterium tumefaciens octopine-type Ti plasmids is closely related to the transfer system of an IncP plasmid and distantly related to Ti plasmid vir genes. J. Bacteriol. 178:4248-4257. - PMC - PubMed
-
- Andersson, R. A., A. R. Eriksson, R. Heikinheimo, A. Mae, M. Pirhonen, V. Koiv, H. Hyytiainen, A. Tuikkala, and E. T. Palva. 2000. Quorum sensing in the plant pathogen Erwinia carotovora subsp. carotovora: the role of expR(Ecc). Mol. Plant-Microbe Interact. 13:384-393. - PubMed
-
- Banfalvi, Z., E. Kondorosi, and A. Kondorosi. 1985. Rhizobium meliloti carries two megaplasmids. Plasmid 13:129-138. - PubMed
-
- Banfalvi, Z., V. Sakanyan, C. Koncz, A. Kiss, I. Dusha, and A. Kondorosi. 1981. Location of nodulation and nitrogen fixation genes on a high molecular weight plasmid of Rhizobium meliloti. Mol. Gen. Genet. 184:318-325. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

