A continuum of anionic charge: structures and functions of D-alanyl-teichoic acids in gram-positive bacteria
- PMID: 14665680
- PMCID: PMC309049
- DOI: 10.1128/MMBR.67.4.686-723.2003
A continuum of anionic charge: structures and functions of D-alanyl-teichoic acids in gram-positive bacteria
Abstract
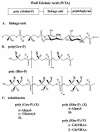
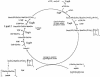
Teichoic acids (TAs) are major wall and membrane components of most gram-positive bacteria. With few exceptions, they are polymers of glycerol-phosphate or ribitol-phosphate to which are attached glycosyl and D-alanyl ester residues. Wall TA is attached to peptidoglycan via a linkage unit, whereas lipoteichoic acid is attached to glycolipid intercalated in the membrane. Together with peptidoglycan, these polymers make up a polyanionic matrix that functions in (i) cation homeostasis; (ii) trafficking of ions, nutrients, proteins, and antibiotics; (iii) regulation of autolysins; and (iv) presentation of envelope proteins. The esterification of TAs with D-alanyl esters provides a means of modulating the net anionic charge, determining the cationic binding capacity, and displaying cations in the wall. This review addresses the structures and functions of D-alanyl-TAs, the D-alanylation system encoded by the dlt operon, and the roles of TAs in cell growth. The importance of dlt in the physiology of many organisms is illustrated by the variety of mutant phenotypes. In addition, advances in our understanding of D-alanyl ester function in virulence and host-mediated responses have been made possible through targeted mutagenesis of dlt. Studies of the mechanism of D-alanylation have identified two potential targets of antibacterial action and provided possible screening reactions for designing novel agents targeted to D-alanyl-TA synthesis.
Figures
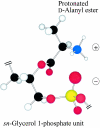


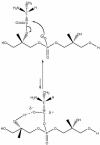


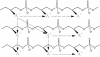
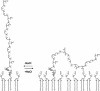
 O distances are both 2.66 Å. In panel B, the corresponding distances are 2.61 and 4.70 Å. In panel A, the carbonyl oxygen and the C-2 proton of glycerol are cis, and in panel B they are trans. Resonance stabilization in the ester linkage determines a rotational barrier (495) between the two conformers. For this figure, the flanking glycerol residues are truncated. The conformations were calculated by the semi-empirical molecular orbital method, MNDO-PM3 (, ; Arnold and Neuhaus, unpublished).
O distances are both 2.66 Å. In panel B, the corresponding distances are 2.61 and 4.70 Å. In panel A, the carbonyl oxygen and the C-2 proton of glycerol are cis, and in panel B they are trans. Resonance stabilization in the ester linkage determines a rotational barrier (495) between the two conformers. For this figure, the flanking glycerol residues are truncated. The conformations were calculated by the semi-empirical molecular orbital method, MNDO-PM3 (, ; Arnold and Neuhaus, unpublished).






References
-
- Aasjord, P., and A. Grov. 1980. Immunoperoxidase and electron microscopy studies of staphylococcal lipoteichoic acid. Acta Pathol. Microbiol. Scand. Sect. B 88:47-52. - PubMed
-
- Abachin, E., C. Poyart, E. Pellegrini, E. Milohanic, F. Fiedler, P. Berche, and P. Trieu-Cuot. 2002. Formation of d-alanyl-lipoteichoic acid is required for adhesion and virulence of Listeria monocytogenes. Mol. Microbiol. 43:1-14. - PubMed
-
- Aly, R., and S. Levit. 1987. Adherence of Staphylococcus aureus to squamous epithelium: role of fibronectin and teichoic acid. Rev. Infect. Dis. 9(Suppl. 4):S341-S350. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

