Increased phosphorylation of the neuronal L-type Ca(2+) channel Ca(v)1.2 during aging
- PMID: 14665691
- PMCID: PMC307685
- DOI: 10.1073/pnas.2236970100
Increased phosphorylation of the neuronal L-type Ca(2+) channel Ca(v)1.2 during aging
Abstract
An increase in Ca2+ influx through L-type Ca2+ channels is thought to contribute to neuronal dysfunctions that underlie senile symptoms and Alzheimer's disease. The molecular basis of the age-dependent up-regulation in neuronal L-type Ca2+ channel activity is largely unknown. We show that phosphorylation of the L-type channel Cav1.2 by cAMP-dependent protein kinase is increased >2-fold in the hippocampus of aged rats. The hippocampus is critical for learning and is one of the first brain regions to be affected in Alzheimer's disease. Phosphorylation of Cav1.2 by cAMP-dependent protein kinase strongly enhances its activity. Therefore, increased Cav1.2 phosphorylation may account for a substantial portion of the age-related rise in neuronal Ca2+ influx and its neuropathological consequences.
Figures
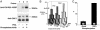

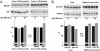
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

