Vascular endothelial growth factor inhibits maturation of dendritic cells induced by lipopolysaccharide, but not by proinflammatory cytokines
- PMID: 14666382
- PMCID: PMC11034272
- DOI: 10.1007/s00262-003-0466-8
Vascular endothelial growth factor inhibits maturation of dendritic cells induced by lipopolysaccharide, but not by proinflammatory cytokines
Abstract
Purpose: Dendritic cells (DCs) play an important role in the host's immunosurveillance against cancer. It has been shown that the function of DCs is impaired and their population decreased in a cancer-bearing host. In the present study, we investigated the mechanism of down-regulation of DCs in a cancer-bearing host.
Methods: We evaluated the relationship between DC infiltration and production of vascular endothelial growth factor (VEGF) in carcinoma tissue by immunohistochemistry. Furthermore, functional and phenotypical alterations of DCs were evaluated when monocyte-derived, mature DCs were treated with VEGF in vitro. Monocyte-derived DCs were generated in a culture of monocyte with interleukin 4 (IL-4) and granulocyte-macrophage colony-stimulating factor, and the maturation of DCs was induced by either lipopolysaccharide (LPS) or a proinflammatory cytokine cocktail: tumor-necrosis factor alpha, prostaglandin E2, IL-6, and IL-1beta.
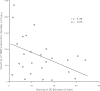
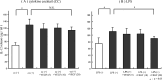
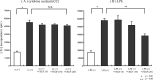
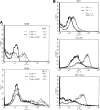
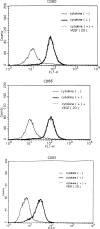
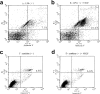
Results: A significant inverse correlation was found between the density of DCs and the quantity of VEGF production in gastric carcinoma tissue (r=-0.39, p<0.05). In LPS-induced maturation, the ability of mature DCs to stimulate allogenic T cells and produce IL-12 (p70 heterodimer) was suppressed by the addition of VEGF in a dose-dependent manner. A lesser expression of costimulatory molecules (CD80 and CD86) was seen in DCs treated with exogenous VEGF than in DCs not treated with VEGF. The population of dead DCs (early and late apoptosis) treated with VEGF increased more than that without VEGF treatment, using the annexin V and propidium iodide evaluation in DCs matured by LPS. In contrast, in DCs matured by the proinflammatory cytokine cocktail, the down-regulation of costimulatory molecules and induction of DC apoptosis was not seen.
Conclusions: These findings show that the inhibition of DC maturation by VEGF differs depending on the maturation status of the DCs.
Figures


Similar articles
-
Vascular endothelial growth factor inhibits the function of human mature dendritic cells mediated by VEGF receptor-2.Cancer Immunol Immunother. 2007 Jun;56(6):761-70. doi: 10.1007/s00262-006-0234-7. Epub 2006 Nov 4. Cancer Immunol Immunother. 2007. PMID: 17086423 Free PMC article.
-
Fas engagement induces the maturation of dendritic cells (DCs), the release of interleukin (IL)-1beta, and the production of interferon gamma in the absence of IL-12 during DC-T cell cognate interaction: a new role for Fas ligand in inflammatory responses.J Exp Med. 2000 Dec 4;192(11):1661-8. doi: 10.1084/jem.192.11.1661. J Exp Med. 2000. PMID: 11104808 Free PMC article.
-
Evaluation of 3 clinical dendritic cell maturation protocols containing lipopolysaccharide and interferon-gamma.J Immunother. 2009 May;32(4):399-407. doi: 10.1097/CJI.0b013e31819e1773. J Immunother. 2009. PMID: 19342965 Free PMC article.
-
Standardized generation of fully mature p70 IL-12 secreting monocyte-derived dendritic cells for clinical use.Cancer Immunol Immunother. 2001 Oct;50(8):417-27. doi: 10.1007/s002620100215. Cancer Immunol Immunother. 2001. PMID: 11726136 Free PMC article.
-
Analysis of Nanoparticle Effects on Maturation of Monocyte Derived Dendritic Cells In Vitro: Version 5.2022 Nov. In: National Cancer Institute’s Nanotechnology Characterization Laboratory Assay Cascade Protocols [Internet]. Bethesda (MD): National Cancer Institute (US); 2005 May 1–. NCL Method ITA-14. 2022 Nov. In: National Cancer Institute’s Nanotechnology Characterization Laboratory Assay Cascade Protocols [Internet]. Bethesda (MD): National Cancer Institute (US); 2005 May 1–. NCL Method ITA-14. PMID: 39012971 Free Books & Documents. Review.
Cited by
-
Transient downregulation of monocyte-derived dendritic-cell differentiation, function, and survival during tumoral progression and regression in an in vivo canine model of transmissible venereal tumor.Cancer Immunol Immunother. 2008 Apr;57(4):479-91. doi: 10.1007/s00262-007-0386-0. Epub 2007 Aug 21. Cancer Immunol Immunother. 2008. PMID: 17710396 Free PMC article.
-
Multifaceted Role of Neuropilins in the Immune System: Potential Targets for Immunotherapy.Front Immunol. 2017 Oct 10;8:1228. doi: 10.3389/fimmu.2017.01228. eCollection 2017. Front Immunol. 2017. PMID: 29067024 Free PMC article. Review.
-
A subanalysis of Japanese patients in a randomized, double-blind, placebo-controlled, phase 3 trial of nivolumab for patients with advanced gastric or gastro-esophageal junction cancer refractory to, or intolerant of, at least two previous chemotherapy regimens (ONO-4538-12, ATTRACTION-2).Gastric Cancer. 2019 Mar;22(2):344-354. doi: 10.1007/s10120-018-0899-6. Epub 2018 Dec 1. Gastric Cancer. 2019. PMID: 30506519 Free PMC article. Clinical Trial.
-
Interleukin-18 has antipermeablity and antiangiogenic activities in the eye: reciprocal suppression with VEGF.J Cell Physiol. 2014 Aug;229(8):974-83. doi: 10.1002/jcp.24575. J Cell Physiol. 2014. PMID: 24515951 Free PMC article.
-
Immunogenicity is preferentially induced in sparse dendritic cell cultures.Sci Rep. 2017 Mar 9;7:43989. doi: 10.1038/srep43989. Sci Rep. 2017. PMID: 28276533 Free PMC article.
References
-
- Choux Int J Cancer. 1997;72:619. - PubMed
-
- Gabrilovich Clin Cancer Res. 1997;3:483. - PubMed
-
- Enk AH, Jonuleit H, Saloga J, Knop J. Dendritic cells as mediators of tumor-induced tolerance in metastatic melanoma. Int J Cancer. 1997;73:309. - PubMed
-
- Almand Clin Cancer Res. 2000;6:1755. - PubMed
-
- Tsujitani Cancer. 1987;59:501. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

