Leukocyte-inspired biodegradable particles that selectively and avidly adhere to inflamed endothelium in vitro and in vivo
- PMID: 14668435
- PMCID: PMC307664
- DOI: 10.1073/pnas.2631433100
Leukocyte-inspired biodegradable particles that selectively and avidly adhere to inflamed endothelium in vitro and in vivo
Abstract
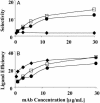
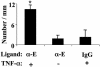
We exploited leukocyte-endothelial cell adhesion chemistry to generate biodegradable particles that exhibit highly selective accumulation on inflamed endothelium in vitro and in vivo. Leukocyte-endothelial cell adhesive particles exhibit up to 15-fold higher adhesion to inflamed endothelium, relative to noninflamed endothelium, under in vitro flow conditions similar to that present in blood vessels, a 6-fold higher adhesion to cytokine inflamed endothelium relative to non-cytokine-treated endothelium in vivo, and a 10-fold enhancement in adhesion to trauma-induced inflamed endothelium in vivo due to the addition of a targeting ligand. The leukocyte-inspired particles have adhesion efficiencies similar to that of leukocytes and were shown to target each of the major inducible endothelial cell adhesion molecules (E-selectin, P-selectin, vascular cell adhesion molecule 1, and intercellular adhesion molecule 1) that are up-regulated at sites of pathological inflammation. The potential for targeted drug delivery to inflamed endothelium has significant implications for the improved treatment of an array of pathologies, including cardiovascular disease, arthritis, inflammatory bowel disease, and cancer.
Figures

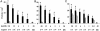
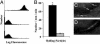
References
-
- Cybulsky, M. I. & Gimbrone, M. A., Jr. (1991) Science 251, 788-791. - PubMed
-
- Soriano, A., Salas, A., Sans, M., Gironella, M., Elena, M., Anderson, D. C., Pique, J. M. & Panes, J. (2000) Lab. Invest. 80, 1541-1551. - PubMed
-
- Jones, S. P., Trocha, S. D., Strange, M. B., Granger, D. N., Kevil, C. G., Bullard, D. C. & Lefer, D. J. (2000) Am. J. Physiol. 279, H2196-H2201. - PubMed
-
- Chapman, P. T., Jamar, F., Keelan, E. T., Peters, A. M. & Haskard, D. O. (1996) Arthritis Rheum. 39, 1371-1375. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

