A unique region of RILP distinguishes it from its related proteins in its regulation of lysosomal morphology and interaction with Rab7 and Rab34
- PMID: 14668488
- PMCID: PMC329395
- DOI: 10.1091/mbc.e03-06-0413
A unique region of RILP distinguishes it from its related proteins in its regulation of lysosomal morphology and interaction with Rab7 and Rab34
Abstract
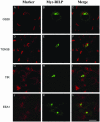
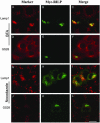
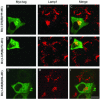
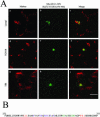
Rab7 and Rab34 are implicated in regulation of lysosomal morphology and they share a common effector referred to as the RILP (Rab-interacting lysosomal protein). Two novel proteins related to RILP were identified and are tentatively referred to as RLP1 and RLP2 (for RILP-like protein 1 and 2, respectively). Overexpression of RILP caused enlarged lysosomes that are positioned more centrally in the cell. However, the morphology and distribution of lysosomes were not affected by overexpression of either RLP1 or RLP2. The molecular basis for the effect of RILP on lysosomes was investigated, leading to the demonstration that a 62-residue region (amino acids 272-333) of RILP is necessary for RILP's role in regulating lysosomal morphology. Remarkably, transferring this 62-residue region unique to RILP into corresponding sites in RLP1 rendered the chimeric protein capable of regulating lysosome morphology. A correlation between the interaction with GTP-bound form of both Rab proteins and the capability of regulating lysosomes was established. These results define a unique region in RILP responsible for its specific role in regulating lysosomal morphology as well as in its interaction with Rab7 and Rab34.
Figures




Similar articles
-
Assay and functional properties of Rab34 interaction with RILP in lysosome morphogenesis.Methods Enzymol. 2005;403:675-87. doi: 10.1016/S0076-6879(05)03058-2. Methods Enzymol. 2005. PMID: 16473629
-
The Rab-interacting lysosomal protein, a Rab7 and Rab34 effector, is capable of self-interaction.Biochem Biophys Res Commun. 2005 Aug 19;334(1):128-33. doi: 10.1016/j.bbrc.2005.06.067. Biochem Biophys Res Commun. 2005. PMID: 15996637
-
Rab-interacting lysosomal protein (RILP): the Rab7 effector required for transport to lysosomes.EMBO J. 2001 Feb 15;20(4):683-93. doi: 10.1093/emboj/20.4.683. EMBO J. 2001. PMID: 11179213 Free PMC article.
-
Rab7: role of its protein interaction cascades in endo-lysosomal traffic.Cell Signal. 2011 Mar;23(3):516-21. doi: 10.1016/j.cellsig.2010.09.012. Epub 2010 Sep 21. Cell Signal. 2011. PMID: 20851765 Review.
-
A new V-ATPase regulatory mechanism mediated by the Rab interacting lysosomal protein (RILP).Commun Integr Biol. 2014 Oct 31;7(5):e971572. doi: 10.4161/cib.29616. eCollection 2014 Oct. Commun Integr Biol. 2014. PMID: 26843904 Free PMC article. Review.
Cited by
-
Autophagy and neurodegeneration: Unraveling the role of C9ORF72 in the regulation of autophagy and its relationship to ALS-FTD pathology.Front Cell Neurosci. 2023 Mar 16;17:1086895. doi: 10.3389/fncel.2023.1086895. eCollection 2023. Front Cell Neurosci. 2023. PMID: 37006471 Free PMC article. Review.
-
Evolutional insights into the interaction between Rab7 and RILP in lysosome motility.iScience. 2023 Jun 7;26(7):107040. doi: 10.1016/j.isci.2023.107040. eCollection 2023 Jul 21. iScience. 2023. PMID: 37534141 Free PMC article.
-
Molecular mechanism of dynein-dynactin complex assembly by LIS1.Science. 2024 Mar 29;383(6690):eadk8544. doi: 10.1126/science.adk8544. Epub 2024 Mar 29. Science. 2024. PMID: 38547289 Free PMC article.
-
Small GTPase Rab40c associates with lipid droplets and modulates the biogenesis of lipid droplets.PLoS One. 2013 Apr 30;8(4):e63213. doi: 10.1371/journal.pone.0063213. Print 2013. PLoS One. 2013. PMID: 23638186 Free PMC article.
-
Rab GTPases: The principal players in crafting the regulatory landscape of endosomal trafficking.Comput Struct Biotechnol J. 2022 Aug 11;20:4464-4472. doi: 10.1016/j.csbj.2022.08.016. eCollection 2022. Comput Struct Biotechnol J. 2022. PMID: 36051867 Free PMC article. Review.
References
-
- Chavrier, P., and Goud, B. (1999). The role of ARF and Rab GTPases in membrane transport. Curr. Opin. Cell Biol. 11, 466-475. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

