Ace revisited: a new target for structure-based drug design
- PMID: 14668810
- PMCID: PMC7097707
- DOI: 10.1038/nrd1227
Ace revisited: a new target for structure-based drug design
Abstract
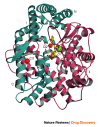
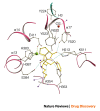
Current-generation angiotensin-converting enzyme (ACE) inhibitors are widely used for cardiovascular diseases, including high blood pressure, heart failure, heart attack and kidney failure, and have combined annual sales in excess of US $6 billion. However, the use of these ACE inhibitors, which were developed in the late 1970s and early 1980s, is hampered by common side effects. Moreover, we now know that ACE actually consists of two parts (called the N- and C-domains) that have different functions. Therefore, the design of specific domain-selective ACE inhibitors is expected to produce next-generation drugs that might be safer and more effective. Here we discuss the structural features of current inhibitors and outline how next-generation ACE inhibitors could be designed by using the three-dimensional molecular structure of human testis ACE. The ACE structure provides a unique opportunity for rational drug design, based on a combination of in silico modelling using existing inhibitors as scaffolds and iterative lead optimization to drive the synthetic chemistry.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

