Matrix metalloproteinase-9 is required for adequate angiogenic revascularization of ischemic tissues: potential role in capillary branching
- PMID: 14670843
- PMCID: PMC6716372
- DOI: 10.1161/01.RES.0000111527.42357.62
Matrix metalloproteinase-9 is required for adequate angiogenic revascularization of ischemic tissues: potential role in capillary branching
Abstract
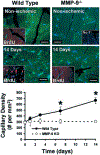
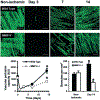
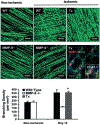
Angiogenesis, an essential component of a variety of physiological and pathological processes, offers attractive opportunities for therapeutic regulation. We hypothesized that matrix metalloproteinase-9 genetic deficiency (MMP-9-/-) will impair angiogenesis triggered by tissue ischemia, induced experimentally by femoral artery ligation in mice. To investigate the role of MMP-9, we performed a series of biochemical and histological analyses, including zymography, simultaneous detection of perfused capillaries, MMP-9 promoter activity, MMP-9 protein, and macrophages in MMP-9-/- and wild-type (WT) mice. We found that ischemia resulted in doubling of capillary density in WT and no change in the MMP-9-/- ischemic tissues, which translated into increased (39%) perfusion capacity only in the WT at 14 days after ligation. We also confirmed that capillaries in the MMP-9-/- presented significantly (P<0.05) less points of capillary intersections, interpreted by us as decreased branching. The combined conclusions from simultaneous localizations of MMP-9 expression, capillaries, and macrophages suggested that macrophage MMP-9 participates in capillary branching. Transplantation of WT bone marrow into the MMP-9-/-, restored capillary branching, further supporting the contribution of bone marrow-derived macrophages in supplying the necessary MMP-9. Our study indicates that angiogenesis triggered by tissue ischemia requires MMP-9, which may be involved in capillary branching, a potential novel role for this MMP that could be exploited to control angiogenesis.
Figures


Comment in
-
Macrophage matrix metalloproteinase-9 regulates angiogenesis in ischemic muscle.Circ Res. 2004 Feb 6;94(2):138-9. doi: 10.1161/01.RES.0000117525.23089.1A. Circ Res. 2004. PMID: 14764648 No abstract available.
Similar articles
-
Temporal expression and activation of matrix metalloproteinases-2, -9, and membrane type 1-matrix metalloproteinase following acute hindlimb ischemia.J Surg Res. 2003 May 1;111(1):8-15. doi: 10.1016/s0022-4804(02)00034-3. J Surg Res. 2003. PMID: 12842442
-
p27(kip1) Knockout enhances collateralization in response to hindlimb ischemia.J Vasc Surg. 2016 May;63(5):1351-9. doi: 10.1016/j.jvs.2014.12.047. Epub 2015 Feb 18. J Vasc Surg. 2016. PMID: 25701497
-
Despite normal arteriogenic and angiogenic responses, hind limb perfusion recovery and necrotic and fibroadipose tissue clearance are impaired in matrix metalloproteinase 9-deficient mice.J Vasc Surg. 2015 Jun;61(6):1583-94.e1-10. doi: 10.1016/j.jvs.2014.01.038. Epub 2014 Feb 28. J Vasc Surg. 2015. PMID: 24582703 Free PMC article.
-
Macrophage matrix metalloproteinase-9 regulates angiogenesis in ischemic muscle.Circ Res. 2004 Feb 6;94(2):138-9. doi: 10.1161/01.RES.0000117525.23089.1A. Circ Res. 2004. PMID: 14764648 No abstract available.
-
Role of neutrophil-derived matrix metalloproteinase-9 in tissue regeneration.Histol Histopathol. 2010 Jun;25(6):765-70. doi: 10.14670/HH-25.765. Histol Histopathol. 2010. PMID: 20376783 Review.
Cited by
-
Pro-angiogenic and anti-inflammatory regulation by functional peptides loaded in polymeric implants for soft tissue regeneration.Tissue Eng Part A. 2013 Feb;19(3-4):437-47. doi: 10.1089/ten.TEA.2012.0158. Epub 2012 Oct 19. Tissue Eng Part A. 2013. PMID: 22953721 Free PMC article.
-
Impaired vascular remodeling after endothelial progenitor cell transplantation in MMP9-deficient mice suffering cortical cerebral ischemia.J Cereb Blood Flow Metab. 2015 Oct;35(10):1547-51. doi: 10.1038/jcbfm.2015.180. Epub 2015 Jul 29. J Cereb Blood Flow Metab. 2015. PMID: 26219597 Free PMC article.
-
The Role of Vascular Endothelial Growth Factor Receptor-1 Signaling in the Recovery from Ischemia.PLoS One. 2015 Jul 2;10(7):e0131445. doi: 10.1371/journal.pone.0131445. eCollection 2015. PLoS One. 2015. PMID: 26133989 Free PMC article.
-
Microvascular anatomy suggests varying aerobic activity levels in the adipose tissues of diving tetrapods.J Comp Physiol B. 2022 Sep;192(5):623-645. doi: 10.1007/s00360-022-01446-5. Epub 2022 Jul 2. J Comp Physiol B. 2022. PMID: 35779114
-
Cerebral ischaemia and matrix metalloproteinase-9 modulate the angiogenic function of early and late outgrowth endothelial progenitor cells.J Cell Mol Med. 2013 Dec;17(12):1543-53. doi: 10.1111/jcmm.12116. Epub 2013 Aug 15. J Cell Mol Med. 2013. PMID: 23945132 Free PMC article.
References
-
- Folkman J Role of angiogenesis in tumor growth and metastasis. Semin Oncol. 2002;29:15–18. - PubMed
-
- Aiello LP. Clinical implications of vascular growth factors in proliferative retinopathies. Curr Opin Ophthalmol. 1997;8:19–31. - PubMed
-
- Ross JS, Stagliano NE, Donovan MJ, Breitbart RE, Ginsburg GS. Atherosclerosis: a cancer of the blood vessels? Am J Clin Pathol. 2001; 116(suppl):S97–S107. - PubMed
-
- Carmeliet P Mechanisms of angiogenesis and arteriogenesis. Nat Med. 2000;6:389–395. - PubMed
-
- Rivilis I, Milkiewicz M, Boyd P, Goldstein J, Brown MD, Egginton S, Hansen FM, Hudlicka O, Haas TL. Differential involvement of MMP-2 and VEGF during muscle stretch- versus shear stress-induced angiogenesis. Am J Physiol Heart Circ Physiol. 2002;283:H1430–H1438. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

