In vivo analysis of cell division, cell growth, and differentiation at the shoot apical meristem in Arabidopsis
- PMID: 14671026
- PMCID: PMC301396
- DOI: 10.1105/tpc.017962
In vivo analysis of cell division, cell growth, and differentiation at the shoot apical meristem in Arabidopsis
Abstract
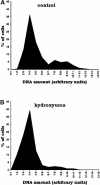
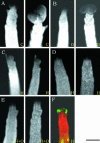
The aerial parts of the plant are generated by groups of rapidly dividing cells called shoot apical meristems. To analyze cell behavior in these structures, we developed a technique to visualize living shoot apical meristems using the confocal microscope. This method, combined with green fluorescent protein marker lines and vital stains, allows us to follow the dynamics of cell proliferation, cell expansion, and cell differentiation at the shoot apex. Using this approach, the effects of several mitotic drugs on meristem development were studied. Oryzalin (depolymerizing microtubules) very rapidly caused cell division arrest. Nevertheless, both cell expansion and cell differentiation proceeded in the treated meristems. Interestingly, DNA synthesis was not blocked, and the meristematic cells went through several rounds of endoreduplication in the presence of the drug. We next treated the meristems with two inhibitors of DNA synthesis, aphidicolin and hydroxyurea. In this case, cell growth and, later, cell differentiation were inhibited, suggesting an important role for DNA synthesis in growth and patterning.
Figures

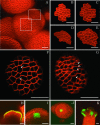

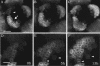
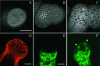
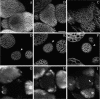


References
-
- Ambros, V. (1999). Cell-cycle dependent sequencing of cell fate decisions in Caenorhabditis elegans vulva precursor cells. Development 126, 1947–1956. - PubMed
-
- Blazquez, M.A., Soowal, L.N., Lee, I., and Weigel, D. (1997). LEAFY expression and flower initiation in Arabidopsis. Development 124, 3835–3844. - PubMed
-
- Blilou, I., Frugier, F., Folmer, S., Serralbo, O., Willemsen, V., Wolkenfelt, H., Eloy, N., Ferreira, P.C.G., Weisbeek, P., and Scheres, B. (2002). The Arabidopsis HOBBIT gene encodes a CDC27 homolog that links the plant cell cycle to progression of cell differentiation. Genes Dev. 16, 2566–2575. - PMC - PubMed
-
- Boisnard-Lorig, C., Colon-Carmona, A., Bauch, M., Hodge, S., Doerner, P., Bancharel, E., Dumas, C., Haseloff, J., and Berger, F. (2001). Dynamic analyses of the expression of the HISTONE:YFP fusion protein in Arabidopsis show that syncytial endosperm is divided in mitotic domains. Plant Cell 13, 495–509. - PMC - PubMed
-
- Bossinger, G., and Smyth, D.R. (1996). Initiation patterns of flower and floral organ development in Arabidopsis thaliana. Development 122, 1093–1102. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

