Cholesterol removal by methyl-beta-cyclodextrin inhibits poliovirus entry
- PMID: 14671085
- PMCID: PMC303393
- DOI: 10.1128/jvi.78.1.33-41.2004
Cholesterol removal by methyl-beta-cyclodextrin inhibits poliovirus entry
Abstract
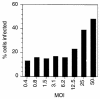
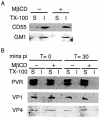
Upon binding to the poliovirus receptor (PVR), the poliovirus 160S particles undergo a conformational transition to generate 135S particles, which are believed to be intermediates in the virus entry process. The 135S particles interact with host cell membranes through exposure of the N termini of VP1 and the myristylated VP4 protein, and successful cytoplasmic delivery of the genomic RNA requires the interaction of these domains with cellular membranes whose identity is unknown. Because detergent-insoluble microdomains (DIMs) in the plasma membrane have been shown to be important in the entry of other picornaviruses, it was of interest to determine if poliovirus similarly required DIMs during virus entry. We show here that methyl-beta-cyclodextrin (MbetaCD), which disrupts DIMs by depleting cells of cholesterol, inhibits virus infection and that this inhibition was partially reversed by partially restoring cholesterol levels in cells, suggesting that MbetaCD inhibition of virus infection was mediated by removal of cellular cholesterol. However, fractionation of cellular membranes into DIMs and detergent-soluble membrane fractions showed that both PVR and poliovirus capsid proteins localize not to DIMs but to detergent-soluble membrane fractions during entry into the cells, and their localization was unaffected by treatment with MbetaCD. We further demonstrate that treatment with MbetaCD inhibits RNA delivery after formation of the 135S particles. These data indicate that the cholesterol status of the cell is important during the process of genome delivery and that these entry pathways are distinct from those requiring DIM integrity.
Figures





References
-
- Andino, R., D. Silvera, S. D. Suggett, P. L. Achacoso, C. J. Miller, D. Baltimore, and M. B. Feinberg. 1994. Engineering poliovirus as a vaccine vector for the expression of diverse antigens. Science 265:1448-1451. - PubMed
-
- Bastiaanse, E. M., K. M. Hold, and A. Van der Laarse. 1997. The effect of membrane cholesterol content on ion transport processes in plasma membranes. Cardiovasc. Res. 33:272-283. - PubMed
-
- Chang, H. M., R. Reitstetter, R. P. Mason, and R. Gruener. 1995. Attenuation of channel kinetics and conductance by cholesterol: an interpretation using structural stress as a unifying concept. J. Membr. Biol. 143:51-63. - PubMed
-
- Chen, X., and M. D. Resh. 2001. Activation of mitogen-activated protein kinase by membrane-targeted Raf chimeras is independent of raft localization. J. Biol. Chem. 276:34617-34623. - PubMed
-
- Chen, X., and M. D. Resh. 2002. Cholesterol depletion from the plasma membrane triggers ligand-independent activation of the epidermal growth factor receptor. J. Biol. Chem. 277:49631-49637. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

