Inhibition of S-phase cyclin-dependent kinase activity blocks expression of Epstein-Barr virus immediate-early and early genes, preventing viral lytic replication
- PMID: 14671092
- PMCID: PMC303396
- DOI: 10.1128/jvi.78.1.104-115.2004
Inhibition of S-phase cyclin-dependent kinase activity blocks expression of Epstein-Barr virus immediate-early and early genes, preventing viral lytic replication
Abstract
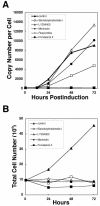
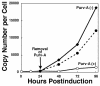
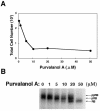
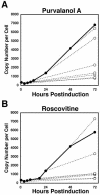
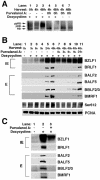
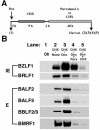
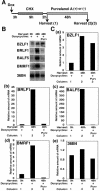
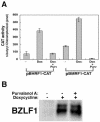
The induction of lytic replication of the Epstein-Barr virus (EBV) completely arrests cell cycle progression, in spite of elevation of S-phase cyclin-dependent kinase (CDK) activity, thereby causing accumulation of hyperphosphorylated forms of retinoblastoma (Rb) protein (A. Kudoh, M. Fujita, T. Kiyono, K. Kuzushima, Y. Sugaya, S. Izuta, Y. Nishiyama, and T. Tsurumi, J. Virol. 77:851-861, 2003). Thus, the EBV lytic program appears to promote specific cell cycle-associated activity involved in the progression from G1 to S phase. We have proposed that this provides a cellular environment that is advantageous for EBV productive infection. Purvalanol A and roscovitine, inhibitors of S-phase CDKs, blocked the viral lytic replication when cells were treated at the early stage of lytic infection, while well-characterized inhibitors of enzymes, such as mitogen-activated protein kinase, phosphatidylinositol 3-kinase, and protein kinase C, known to be involved in BZLF1 gene expression did not. Inhibition of CDK activity resulted in the accumulation of the hypophosphorylated form of Rb protein and inhibition of expression of EBV immediate-early and early proteins. Cycloheximide block-and-release experiments clearly demonstrated that even in the presence of enough amounts of the BZLF1 protein, purvalanol A blocked expression of lytic viral proteins at transcription level. Furthermore, reporter gene experiments confirmed that BZLF1-induced activation of early EBV promoters was impaired in the presence of the CDK inhibitor. We conclude here that the EBV lytic program promotes specific cell cycle-associated activity involved in the progression from G1 to S phase because the S-phase-like cellular environment is essential for the expression of immediate-early and early genes supplying the viral replication proteins and hence for lytic viral replication.
Figures
Similar articles
-
Reactivation of lytic replication from B cells latently infected with Epstein-Barr virus occurs with high S-phase cyclin-dependent kinase activity while inhibiting cellular DNA replication.J Virol. 2003 Jan;77(2):851-61. doi: 10.1128/jvi.77.2.851-861.2003. J Virol. 2003. PMID: 12502801 Free PMC article.
-
Fatty acid synthase expression is induced by the Epstein-Barr virus immediate-early protein BRLF1 and is required for lytic viral gene expression.J Virol. 2004 Apr;78(8):4197-206. doi: 10.1128/jvi.78.8.4197-4206.2004. J Virol. 2004. PMID: 15047835 Free PMC article.
-
Protein kinase C-independent activation of the Epstein-Barr virus lytic cycle.J Virol. 2002 Jun;76(11):5612-26. doi: 10.1128/jvi.76.11.5612-5626.2002. J Virol. 2002. PMID: 11991990 Free PMC article.
-
Latent and lytic Epstein-Barr virus replication strategies.Rev Med Virol. 2005 Jan-Feb;15(1):3-15. doi: 10.1002/rmv.441. Rev Med Virol. 2005. PMID: 15386591 Review.
-
Lytic cycle switches of oncogenic human gammaherpesviruses.Adv Cancer Res. 2007;97:81-109. doi: 10.1016/S0065-230X(06)97004-3. Adv Cancer Res. 2007. PMID: 17419942 Review.
Cited by
-
Kaposi's sarcoma-associated herpesvirus-encoded protein kinase and its interaction with K-bZIP.J Virol. 2007 Feb;81(3):1072-82. doi: 10.1128/JVI.01473-06. Epub 2006 Nov 15. J Virol. 2007. PMID: 17108053 Free PMC article.
-
Viral-Targeted Strategies Against EBV-Associated Lymphoproliferative Diseases.Front Oncol. 2019 Feb 26;9:81. doi: 10.3389/fonc.2019.00081. eCollection 2019. Front Oncol. 2019. PMID: 30873380 Free PMC article. Review.
-
Phosphoproteomic analyses reveal signaling pathways that facilitate lytic gammaherpesvirus replication.PLoS Pathog. 2013 Sep;9(9):e1003583. doi: 10.1371/journal.ppat.1003583. Epub 2013 Sep 19. PLoS Pathog. 2013. PMID: 24068923 Free PMC article.
-
Noise cancellation: viral fine tuning of the cellular environment for its own genome replication.PLoS Pathog. 2010 Dec 16;6(12):e1001158. doi: 10.1371/journal.ppat.1001158. PLoS Pathog. 2010. PMID: 21187893 Free PMC article. Review.
-
Critical reanalysis of the methods that discriminate the activity of CDK2 from CDK1.Cell Cycle. 2016 May 2;15(9):1184-8. doi: 10.1080/15384101.2016.1160983. Epub 2016 Mar 17. Cell Cycle. 2016. PMID: 26986210 Free PMC article. Review.
References
-
- Adams, P. D., and W. G. Kaelin, Jr. 1995. Transcriptional control by E2F. Semin. Cancer Biol. 6:99-108. - PubMed
-
- Adamson, A. L., and S. C. Kenney. 1998. Rescue of the Epstein-Barr virus BZLF1 mutant, Z(S186A), early gene activation defect by the BRLF1 gene product. Virology 251:187-197. - PubMed
-
- Borgne, A., and L. Meijer. 1996. Sequential dephosphorylation of p34(cdc2) on Thr-14 and Tyr-15 at the prophase/metaphase transition. J. Biol. Chem. 271:27847-27854. - PubMed
-
- Bresnahan, W. A., I. Boldogh, P. Chi, E. A. Thompson, and T. Albrecht. 1997. Inhibition of cellular Cdk2 activity blocks human cytomegalovirus replication. Virology 231:239-247. - PubMed
-
- Bresnahan, W. A., I. Boldogh, E. A. Thompson, and T. Albrecht. 1996. Human cytomegalovirus inhibits cellular DNA synthesis and arrests productively infected cells in late G1. Virology 224:150-160. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

