Evidence for an essential catalytic role of the F10 protein kinase in vaccinia virus morphogenesis
- PMID: 14671107
- PMCID: PMC303407
- DOI: 10.1128/jvi.78.1.257-265.2004
Evidence for an essential catalytic role of the F10 protein kinase in vaccinia virus morphogenesis
Abstract
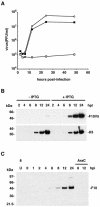
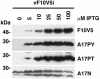
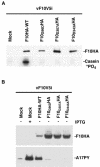
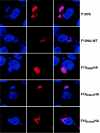
Temperature-sensitive mutants of vaccinia virus, with genetic changes that map to the open reading frame encoding the F10 protein kinase, exhibit a defect at an early stage of viral morphogenesis. To further study the role of the enzyme, we constructed recombinant vaccinia virus vF10V5i, which expresses inducible V5 epitope-tagged F10 and is dependent on a chemical inducer for plaque formation and replication. In the absence of inducer, viral membrane formation was delayed and crescents and occasional immature forms were detected only late in infection. When the temperature was raised from 37 to 39 degrees C, the block in membrane formation persisted throughout the infection. The increased stringency may be explained by a mild temperature sensitivity of the wild-type F10 kinase, which reduced the activity of the very small amount expressed in the absence of inducer, or by the thermolability of an unphosphorylated kinase substrate or uncomplexed F10-interacting protein. Further analyses demonstrated that tyrosine and threonine phosphorylation of the A17 membrane component was inhibited in the absence of inducer. The phosphorylation defect could be overcome by transfection of plasmids that express wild-type F10, but not by plasmids that express F10 with single amino acid substitutions that abolished catalytic activity. Although the mutated forms of F10 were stable and concentrated in viral factories, only the wild-type protein complemented the assembly and replication defects of vF10V5i in the absence of inducer. These studies provide evidence for an essential catalytic role of the F10 kinase in vaccinia virus morphogenesis.
Figures

References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

