Physical and functional interactions between vaccinia virus F10 protein kinase and virion assembly proteins A30 and G7
- PMID: 14671108
- PMCID: PMC303406
- DOI: 10.1128/jvi.78.1.266-274.2004
Physical and functional interactions between vaccinia virus F10 protein kinase and virion assembly proteins A30 and G7
Abstract
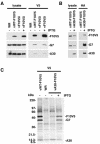
An early step in vaccinia virus morphogenesis, the association of crescent membranes with electron-dense granular material, is perturbed when expression of the viral protein encoded by the A30L or G7L open reading frame is repressed. Under these conditions, we found that phosphorylation of the A17 membrane protein, which is mediated by the F10 kinase, was severely reduced. Furthermore, A30 and G7 stimulated F10-dependent phosphorylation of A17 in the absence of other viral late proteins. Evidence for physical interactions between A30, G7, and F10 was obtained by their coimmunoprecipitation with antibody against A30 or F10. In addition, phosphorylation of A30 was dependent on the F10 kinase and autophosphorylation of F10 was stimulated by A30 and G7. Nevertheless, the association of A30, G7, and F10 occurred even with mutated, catalytically inactive forms of F10. Just as A30 and G7 are mutually dependent on each other for stability, F10 was nearly undetectable in the absence of A30 and G7. The reverse is not true, however, as repression of F10 did not diminish A30 or G7. Interaction of F10 with A30 and G7 presumably occurred within the virus factory areas of the cytoplasm, where each was concentrated. F10 localized predominantly in the cortical region of immature virions, beneath the membrane where A17 is located. F10 remained associated with the particulate core fraction of mature virions after treatment with a nonionic detergent and reducing agent. The formation of protein complexes such as the one involving A30, G7, and F10 may be a mechanism for the regulated packaging and processing of virion components.
Figures








Similar articles
-
Genetic and cell biological characterization of the vaccinia virus A30 and G7 phosphoproteins.J Virol. 2005 Jun;79(11):7146-61. doi: 10.1128/JVI.79.11.7146-7161.2005. J Virol. 2005. PMID: 15890954 Free PMC article.
-
A complex of seven vaccinia virus proteins conserved in all chordopoxviruses is required for the association of membranes and viroplasm to form immature virions.Virology. 2004 Dec 20;330(2):447-59. doi: 10.1016/j.virol.2004.10.008. Virology. 2004. PMID: 15567438
-
Vaccinia virus G7L protein Interacts with the A30L protein and is required for association of viral membranes with dense viroplasm to form immature virions.J Virol. 2003 Mar;77(6):3418-29. doi: 10.1128/jvi.77.6.3418-3429.2003. J Virol. 2003. PMID: 12610117 Free PMC article.
-
In a nutshell: structure and assembly of the vaccinia virion.Adv Virus Res. 2006;66:31-124. doi: 10.1016/S0065-3527(06)66002-8. Adv Virus Res. 2006. PMID: 16877059 Review.
-
The formation and function of extracellular enveloped vaccinia virus.J Gen Virol. 2002 Dec;83(Pt 12):2915-2931. doi: 10.1099/0022-1317-83-12-2915. J Gen Virol. 2002. PMID: 12466468 Review.
Cited by
-
Vaccinia virus mutants with alanine substitutions in the conserved G5R gene fail to initiate morphogenesis at the nonpermissive temperature.J Virol. 2004 Oct;78(19):10238-48. doi: 10.1128/JVI.78.19.10238-10248.2004. J Virol. 2004. PMID: 15367589 Free PMC article.
-
Association of the vaccinia virus A11 protein with the endoplasmic reticulum and crescent precursors of immature virions.J Virol. 2013 Sep;87(18):10195-206. doi: 10.1128/JVI.01601-13. Epub 2013 Jul 17. J Virol. 2013. PMID: 23864611 Free PMC article.
-
Vaccinia virus nonstructural protein encoded by the A11R gene is required for formation of the virion membrane.J Virol. 2005 Jun;79(11):6598-609. doi: 10.1128/JVI.79.11.6598-6609.2005. J Virol. 2005. PMID: 15890898 Free PMC article.
-
A guide to viral inclusions, membrane rearrangements, factories, and viroplasm produced during virus replication.Adv Virus Res. 2007;70:101-82. doi: 10.1016/S0065-3527(07)70004-0. Adv Virus Res. 2007. PMID: 17765705 Free PMC article. Review.
-
Cell biological and functional characterization of the vaccinia virus F10 kinase: implications for the mechanism of virion morphogenesis.J Virol. 2005 Feb;79(4):2171-90. doi: 10.1128/JVI.79.4.2171-2190.2005. J Virol. 2005. PMID: 15681420 Free PMC article.
References
-
- Earl, P. L., N. Cooper, S. Wyatt, B. Moss, and M. W. Carroll. 1998. Preparation of cell cultures and vaccinia virus stocks, p. 16.16.1-16.16.3. In F. M. Ausubel, R. Brent, R. E. Kingston, D. D. Moore, J. G. Seidman, J. A. Smith, and K. Struhl (ed.), Current protocols in molecular biology, vol. 2. John Wiley and Sons, New York, N.Y.
-
- Earl, P. L., and B. Moss. 1998. Characterization of recombinant vaccinia viruses and their products, p. 16.18.1-16.18.11. In F. M. Ausubel, R. Brent, R. E. Kingston, D. D. Moore, J. G. Seidman, J. A. Smith, and K. Struhl (ed.), Current protocols in molecular biology, vol. 2. John Wiley and Sons, New York, N.Y. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

