Tetracycline-inducible packaging cell line for production of flavivirus replicon particles
- PMID: 14671135
- PMCID: PMC303381
- DOI: 10.1128/jvi.78.1.531-538.2004
Tetracycline-inducible packaging cell line for production of flavivirus replicon particles
Abstract
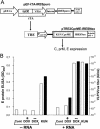
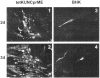
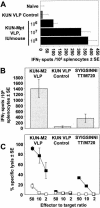
We have previously developed replicon vectors derived from the Australian flavivirus Kunjin that have a unique noncytopathic nature and have been shown to direct prolonged high-level expression of encoded heterologous genes in vitro and in vivo and to induce strong and long-lasting immune responses to encoded immunogens in mice. To facilitate further applications of these vectors in the form of virus-like particles (VLPs), we have now generated a stable BHK packaging cell line, tetKUNCprME, carrying a Kunjin structural gene cassette under the control of a tetracycline-inducible promoter. Withdrawal of tetracycline from the medium resulted in production of Kunjin structural proteins that were capable of packaging transfected and self-amplified Kunjin replicon RNA into the secreted VLPs at titers of up to 1.6 x 10(9) VLPs per ml. Furthermore, secreted KUN replicon VLPs from tetKUNCprME cells could be harvested continuously for as long as 10 days after RNA transfection, producing a total yield of more than 10(10) VLPs per 10(6) transfected cells. Passaging of VLPs on Vero cells or intracerebral injection into 2- to 4-day-old suckling mice illustrated the complete absence of any infectious Kunjin virus. tetKUNCprME cells were also capable of packaging replicon RNA from closely and distantly related flaviviruses, West Nile virus and dengue virus type 2, respectively. The utility of high-titer KUN replicon VLPs was demonstrated by showing increasing CD8(+)-T-cell responses to encoded foreign protein with increasing doses of KUN VLPs. A single dose of 2.5 x 10(7) VLPs carrying the human respiratory syncytial virus M2 gene induced 1,400 CD8 T cells per 10(6) splenocytes in an ex vivo gamma interferon enzyme-linked immunospot assay. The packaging cell line thus represents a significant advance in the development of the noncytopathic Kunjin virus replicon-based gene expression system and may be widely applicable to the basic studies of flavivirus RNA packaging and virus assembly as well as to the development of gene expression systems based on replicons from different flaviviruses.
Figures
References
-
- Coia, G., M. D. Parker, G. Speight, M. E. Byrne, and E. G. Westaway. 1988. Nucleotide and complete amino acid sequences of Kunjin virus: definitive gene order and characteristics of the virus-specified proteins. J. Gen. Virol. 69:1-21. - PubMed
-
- Elliott, S. L., S. Pye, T. Le, L. Mateo, J. Cox, L. Macdonald, A. A. Scalzo, C. A. Forbes, and A. Suhrbier. 1999. Peptide based cytotoxic T-cell vaccines; delivery of multiple epitopes, help, memory and problems. Vaccine 17:2009-2019. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

