Autoubiquitylation of the V(D)J recombinase protein RAG1
- PMID: 14671314
- PMCID: PMC307587
- DOI: 10.1073/pnas.2637012100
Autoubiquitylation of the V(D)J recombinase protein RAG1
Abstract
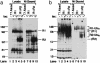
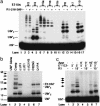
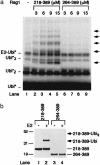
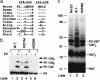
V(D)J recombination, the rearrangement of gene segments to assemble Ig and T cell receptor coding regions, is vital to B and T lymphocyte development. Here, we demonstrate that the V(D)J recombinase protein RAG1 undergoes ubiquitylation in cells. In vitro, the RING finger domain of RAG1 acts as a ubiquitin ligase that mediates its own ubiquitylation at a highly conserved K residue in the RAG1 amino-terminal region. Ubiquitylation is best supported by a specific ubiquitin-conjugating enzyme, UbcH3/CDC34, and requires an intact RAG1 RING finger motif. Disruption of the RING finger and certain RAG1 N-terminal truncations are associated with immunodeficiency in human patients, suggesting that RAG1's ubiquitin ligase is required for its biological role in lymphocyte development.
Figures

Similar articles
-
The roles of the RAG1 and RAG2 "non-core" regions in V(D)J recombination and lymphocyte development.Arch Immunol Ther Exp (Warsz). 2009 Mar-Apr;57(2):105-16. doi: 10.1007/s00005-009-0011-3. Epub 2009 Mar 31. Arch Immunol Ther Exp (Warsz). 2009. PMID: 19333736 Review.
-
The RAG1 V(D)J recombinase/ubiquitin ligase promotes ubiquitylation of acetylated, phosphorylated histone 3.3.Immunol Lett. 2011 May;136(2):156-62. doi: 10.1016/j.imlet.2011.01.005. Epub 2011 Jan 20. Immunol Lett. 2011. PMID: 21256161
-
Correlation between recombinase activating gene 1 ubiquitin ligase activity and V(D)J recombination.Immunology. 2009 Oct;128(2):206-17. doi: 10.1111/j.1365-2567.2009.03101.x. Immunology. 2009. PMID: 19740377 Free PMC article.
-
Role of RAG1 autoubiquitination in V(D)J recombination.Proc Natl Acad Sci U S A. 2015 Jul 14;112(28):8579-83. doi: 10.1073/pnas.1510464112. Epub 2015 Jun 29. Proc Natl Acad Sci U S A. 2015. PMID: 26124138 Free PMC article.
-
Riches in RAGs: Revealing the V(D)J Recombinase through High-Resolution Structures.Trends Biochem Sci. 2017 Jan;42(1):72-84. doi: 10.1016/j.tibs.2016.10.003. Epub 2016 Nov 5. Trends Biochem Sci. 2017. PMID: 27825771 Free PMC article. Review.
Cited by
-
SUMO modification of human XRCC4 regulates its localization and function in DNA double-strand break repair.Mol Cell Biol. 2006 Mar;26(5):1786-94. doi: 10.1128/MCB.26.5.1786-1794.2006. Mol Cell Biol. 2006. PMID: 16478998 Free PMC article.
-
The Arabidopsis thaliana homolog of yeast BRE1 has a function in cell cycle regulation during early leaf and root growth.Plant Cell. 2007 Feb;19(2):417-32. doi: 10.1105/tpc.106.041319. Epub 2007 Feb 28. Plant Cell. 2007. PMID: 17329565 Free PMC article.
-
A WW-like module in the RAG1 N-terminal domain contributes to previously unidentified protein-protein interactions.Nucleic Acids Res. 2009 Jun;37(10):3301-9. doi: 10.1093/nar/gkp192. Epub 2009 Mar 25. Nucleic Acids Res. 2009. PMID: 19324890 Free PMC article.
-
VprBP binds full-length RAG1 and is required for B-cell development and V(D)J recombination fidelity.EMBO J. 2012 Feb 15;31(4):945-58. doi: 10.1038/emboj.2011.455. Epub 2011 Dec 13. EMBO J. 2012. PMID: 22157821 Free PMC article.
-
The CRL4VPRBP(DCAF1) E3 ubiquitin ligase directs constitutive RAG1 degradation in a non-lymphoid cell line.PLoS One. 2021 Oct 14;16(10):e0258683. doi: 10.1371/journal.pone.0258683. eCollection 2021. PLoS One. 2021. PMID: 34648572 Free PMC article.
References
-
- Gellert, M. (2002) Annu. Rev. Biochem. 71, 101–132. - PubMed
-
- Mizuta, R., Mizuta, M., Araki, S. & Kitamura, D. (2002) J. Biol. Chem. 277, 41423–41427. - PubMed
-
- Oettinger, M. A., Schatz, D. G., Gorka, C. & Baltimore, D. (1990) Science 248, 1517–1523. - PubMed
-
- Schatz, D. G., Oettinger, M. A. & Baltimore, D. (1989) Cell 59, 1035–1048. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

