Mullerian Inhibiting Substance inhibits cervical cancer cell growth via a pathway involving p130 and p107
- PMID: 14671316
- PMCID: PMC307614
- DOI: 10.1073/pnas.2636900100
Mullerian Inhibiting Substance inhibits cervical cancer cell growth via a pathway involving p130 and p107
Abstract
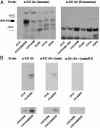
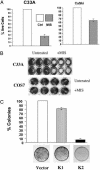
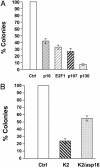
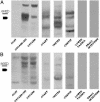
In addition to causing regression of the Mullerian duct in the male embryo, Mullerian Inhibiting Substance (MIS) inhibits the growth of epithelial ovarian cancer cells, which are known to be of Mullerian origin. Because the uterine cervix is derived from the same Mullerian duct precursor as the epithelium of the ovary, we tested the hypothesis that cervical cancer cells might also respond to MIS. A number of cervical cancer cell lines express the MIS type II receptor, and MIS inhibits the growth of both human papilloma virus-transformed and non-human papilloma virus-transformed cervical cell lines, with a more dramatic effect seen in the latter. As in the ovarian cancer cell line OVCAR8, suppression of growth of the C33A cervical cancer cell line by MIS is associated with induction of the p16 tumor suppressor protein. However, in contrast to OVCAR8 cells, induction of p130 and p107 appears to play an important role in the inhibition of growth of C33A cells by MIS. Finally, normal cervical tissue expresses the MIS type II receptor in vivo, supporting the idea that MIS could be a targeted therapy for cervical cancer.
Figures

References
-
- Donahoe, P. K., Swann, D. A., Hayashi, A. & Sullivan, M. D. (1979) Science 205, 913–915. - PubMed
-
- Fuller, A. F., Jr., Guy, S., Budzik, G. P. & Donahoe, P. K. (1982) J. Clin. Endocrinol. Metab. 54, 1051–1055. - PubMed
-
- Fuller, A. F., Jr., Budzik, G. P., Krane, I. M. & Donahoe, P. K. (1984) Gynecol. Oncol. 17, 124–132. - PubMed
-
- Masiakos, P. T., MacLaughlin, D. T., Maheswaran, S., Teixeira, J., Fuller, A. F., Jr., Shah, P. C., Kehas, D. J., Kenneally, M. K., Dombkowski, D. M., Ha, T. U., et al. (1999) Clin. Cancer Res. 5, 3488–3499. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

