Activation of latent myostatin by the BMP-1/tolloid family of metalloproteinases
- PMID: 14671324
- PMCID: PMC307655
- DOI: 10.1073/pnas.2534946100
Activation of latent myostatin by the BMP-1/tolloid family of metalloproteinases
Abstract
Myostatin is a transforming growth factor beta family member that acts as a negative regulator of skeletal muscle growth. Myostatin circulates in the blood of adult mice in a noncovalently held complex with other proteins, including its propeptide, which maintain the C-terminal dimer in a latent, inactive state. This latent form of myostatin can be activated in vitro by treatment with acid; however, the mechanisms by which latent myostatin is activated in vivo are unknown. Here, we show that members of the bone morphogenetic protein-1/tolloid (BMP-1/TLD) family of metalloproteinases can cleave the myostatin propeptide in this complex and can thereby activate latent myostatin. Furthermore, we show that a mutant form of the propeptide resistant to cleavage by BMP-1/TLD proteinases can cause significant increases in muscle mass when injected into adult mice. These findings raise the possibility that members of the BMP-1/TLD family may be involved in activating latent myostatin in vivo and that molecules capable of inhibiting these proteinases may be effective agents for increasing muscle mass for both human therapeutic and agricultural applications.
Figures
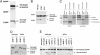
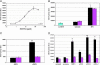

References
-
- McPherron, A. C., Lawler, A. M. & Lee, S.-J. (1997) Nature 387, 83-90. - PubMed
-
- Grobet, L., Martin, L. J. R., Poncelet, D., Pirottin, D., Brouwers, B., Riquet, J., Schoeberlein, A., Dunner, S., Ménissier, F., Massabanda, J., et al. (1997) Nat. Genet. 17, 71-74. - PubMed
-
- Kambadur, R., Sharma, M., Smith, T. P. L. & Bass, J. J. (1997) Genome Res. 7, 910-915. - PubMed
-
- Grobet, L., Poncelet, D., Royo, L. J., Brouwers, B., Pirottin, D., Michaux, C., Ménissier, F., Zanotti, M., Dunner, S. & Georges, M. (1998) Mamm. Genome 9, 210-213. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

