Combination of a zinc finger and homeodomain required for protein-interaction
- PMID: 14672405
- PMCID: PMC3675762
- DOI: 10.1023/a:1026330907065
Combination of a zinc finger and homeodomain required for protein-interaction
Abstract
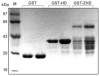
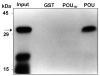
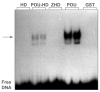
The Zinc Finger Homeodomain Enhancer-binding Protein (Zfhep) is involved in skeletal patterning, immune cell, muscle, and brain development, and is necessary for life. Zfhep contains a single central homeodomain (HD) adjacent to an isolated zinc finger, the function of which is unknown. The placement of a zinc finger so close to a homeodomain is novel in nature. The aim of this work was to characterize the Zfhep homeodomain (HD) or the zinc finger homeodomain (ZHD), with respect to DNA-binding and protein-protein interactions. Glutathione-S-transferase (GST) fusion proteins containing either just the HD or both the zinc finger and HD (ZHD) were expressed in E. coli. The GST fusion protein affinity-binding assay demonstrated that Zfhep ZHD interacts specifically with the POU domain of the Oct-1 transcription factor. The adjacent zinc finger is required since Zfhep HD alone does not interact with Oct-1 POU domain. Furthermore, ZHD does not bind to the POU homeodomain lacking the POU specific region. These results demonstrate that the Zfhep zinc finger homeodomain motif functions as a protein-binding domain in vitro, and suggests that Zfhep may modulate the activity of POU domain transcription factors. However, neither the Zfhep ZHD nor the HD bound DNA in EMSA or selected a DNA-binding site from a pool of random oligonucleotides. This is the first demonstration of a function for the HD region of Zfhep, which is the first case of a bi-partite domain requiring both a zinc finger and a HD for binding to protein.
Figures
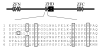





Similar articles
-
Alternative splicing gives rise to two isoforms of Zfhep, a zinc finger/homeodomain protein that binds T3-response elements.DNA Cell Biol. 1996 Aug;15(8):643-51. doi: 10.1089/dna.1996.15.643. DNA Cell Biol. 1996. PMID: 8769566
-
POU domain factors in neural development.Adv Exp Med Biol. 1998;449:39-53. doi: 10.1007/978-1-4615-4871-3_4. Adv Exp Med Biol. 1998. PMID: 10026784 Review.
-
A zinc finger homeodomain transcription factor binds specific thyroid hormone response elements.Mol Cell Endocrinol. 1998 Apr 30;139(1-2):25-35. doi: 10.1016/s0303-7207(98)00076-8. Mol Cell Endocrinol. 1998. PMID: 9705071
-
Cell-specific phosphorylation of Zfhep transcription factor.Biochem Biophys Res Commun. 2002 Aug 16;296(2):368-73. doi: 10.1016/s0006-291x(02)00880-x. Biochem Biophys Res Commun. 2002. PMID: 12163027 Free PMC article.
-
Zinc Finger-Homeodomain and Mini Zinc Finger proteins are key players in plant growth and responses to environmental stresses.J Exp Bot. 2022 Aug 11;73(14):4662-4673. doi: 10.1093/jxb/erac194. J Exp Bot. 2022. PMID: 35536651 Review.
Cited by
-
Intrinsic Balance between ZEB Family Members Is Important for Melanocyte Homeostasis and Melanoma Progression.Cancers (Basel). 2020 Aug 11;12(8):2248. doi: 10.3390/cancers12082248. Cancers (Basel). 2020. PMID: 32796736 Free PMC article. Review.
-
Nuclear import and DNA binding of the ZHD5 transcription factor is modulated by a competitive peptide inhibitor in Arabidopsis.J Biol Chem. 2011 Jan 14;286(2):1659-68. doi: 10.1074/jbc.M110.167692. Epub 2010 Nov 8. J Biol Chem. 2011. PMID: 21059647 Free PMC article.
-
Expanding roles of ZEB factors in tumorigenesis and tumor progression.Am J Cancer Res. 2011;1(7):897-912. Epub 2011 Aug 20. Am J Cancer Res. 2011. PMID: 22016835 Free PMC article.
-
Evolutionary functional analysis and molecular regulation of the ZEB transcription factors.Cell Mol Life Sci. 2012 Aug;69(15):2527-41. doi: 10.1007/s00018-012-0935-3. Epub 2012 Feb 21. Cell Mol Life Sci. 2012. PMID: 22349261 Free PMC article. Review.
-
Phosphorylation Regulates Functions of ZEB1 Transcription Factor.J Cell Physiol. 2016 Oct;231(10):2205-17. doi: 10.1002/jcp.25338. Epub 2016 Mar 10. J Cell Physiol. 2016. PMID: 26868487 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
