Effects of neurotoxic and neuroprotective agents on peripheral nerve regeneration assayed by time-lapse imaging in vivo
- PMID: 14673013
- PMCID: PMC6740511
- DOI: 10.1523/JNEUROSCI.23-36-11479.2003
Effects of neurotoxic and neuroprotective agents on peripheral nerve regeneration assayed by time-lapse imaging in vivo
Abstract
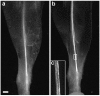
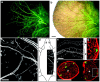
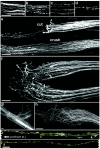
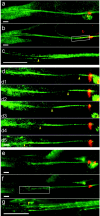
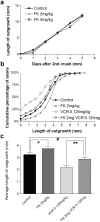
A direct histological assay of axonal regeneration would have many advantages over currently available behavioral, electrophysiological, and radiometric assays. We show that peripheral sensory axons marked with the yellow fluorescent protein in transgenic mice can be viewed transcutaneously in superficial nerves. Degenerating and regenerating axons can be followed in live animals with a dissecting microscope and then, after fixation, studied at high resolution by confocal microscopy. Using this approach, we document differences in regenerative ability after nerve transection, crush injury, and crush injury after a previous "conditioning" lesion. We also show that the chemotherapeutic drug vincristine rapidly but transiently blocks regeneration and that the immunosuppressive drug FK506 modestly enhances regeneration. Moreover, FK506 nearly restores normal regenerative ability in animals treated with submaximal doses of vincristine. Because neuropathy is the major dose-limiting side effect of vincristine, we propose that its efficacy could be enhanced by coadministration of FK506 analogs that are neuroactive but not immunosuppressive.
Figures



References
-
- Aley KO, Reichling DB, Levine JD ( 1996) Vincristine hyperalgesia in the rat: a model of painful vincristine neuropathy in humans. Neuroscience 73: 259-265. - PubMed
-
- Apfel SC, Arezzo JC, Lewis ME, Kessler JA ( 1993) The use of insulin-like growth factor I in the prevention of vincristine neuropathy in mice. Ann NY Acad Sci 692: 243-245. - PubMed
-
- Bignami A, Dahl D, Nguyen BT, Crosby CJ ( 1981) The fate of axonal debris in Wallerian degeneration of rat optic and sciatic nerves. Electron microscopy and immunofluorescence studies with neurofilament antisera. J Neuropathol Exp Neurol 40: 537-550. - PubMed
-
- Boyd JG, Gordon T ( 2003) Neurotrophic factors and their receptors in axonal regeneration and functional recovery after peripheral nerve injury. Mol Neurobiol 27: 277-324. - PubMed
-
- Bradley WG, Lassman LP, Pearce GW, Walton JN ( 1970) The neuromyopathy of vincristine in man. Clinical, electrophysiological and pathological studies. J Neurol Sci 10: 107-131. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
