Gi protein activation in intact cells involves subunit rearrangement rather than dissociation
- PMID: 14673086
- PMCID: PMC307695
- DOI: 10.1073/pnas.2536719100
Gi protein activation in intact cells involves subunit rearrangement rather than dissociation
Abstract
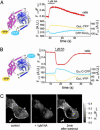
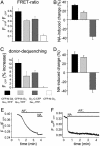
G protein-coupled receptors transduce diverse extracellular signals, such as neurotransmitters, hormones, chemokines, and sensory stimuli, into intracellular responses through activation of heterotrimeric G proteins. G proteins play critical roles in determining specificity and kinetics of subsequent biological responses by modulation of effector proteins. We have developed a fluorescence resonance energy transfer (FRET)-based assay to directly measure mammalian G protein activation in intact cells and found that Gi proteins activate within 1-2 s, which is considerably slower than activation kinetics of the receptors themselves. More importantly, FRET measurements demonstrated that Galphai- and Gbetagamma-subunits do not dissociate during activation, as has been previously postulated. Based on FRET measurements between Galphai-yellow fluorescent protein and Gbetagamma-subunits that were fused to cyan fluorescent protein at various positions, we conclude that, instead, G protein subunits undergo a molecular rearrangement during activation. The detection of a persistent heterotrimeric composition during G protein activation will impact the understanding of how G proteins achieve subtype-selective coupling to effectors. This finding will be of particular interest for unraveling Gbetagamma-induced signaling pathways.
Figures



References
-
- Wall, M. A., Coleman, D. E., Lee, E., Iniguez-Lluhi, J. A., Posner, B. A., Gilman, A. G. & Sprang, S. R. (1995) Cell 83, 1047-1058. - PubMed
-
- Lambright, D. G., Sondek, J., Bohm, A., Skiba, N. P., Hamm, H. E. & Sigler, P. B. (1996) Nature 379, 311-319. - PubMed
-
- Mixon, M. B., Lee, E., Coleman, D. E., Berghuis, A. M., Gilman, A. G. & Sprang, S. R. (1995) Science 270, 954-960. - PubMed
-
- Watson, N., Linder, M. E., Druey, K. M., Kehrl, J. H. & Blumer, K. J. (1996) Nature 383, 172-175. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

