A network of immediate early gene products propagates subtle differences in mitogen-activated protein kinase signal amplitude and duration
- PMID: 14673150
- PMCID: PMC303364
- DOI: 10.1128/MCB.24.1.144-153.2004
A network of immediate early gene products propagates subtle differences in mitogen-activated protein kinase signal amplitude and duration
Abstract
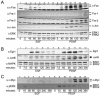
The strength and duration of mitogen-activated protein kinase (MAPK) signaling have been shown to regulate cell fate in different cell types. In this study, a general mechanism is described that explains how subtle differences in signaling kinetics are translated into a specific biological outcome. In fibroblasts, the expression of immediate early gene (IEG)-encoded Fos, Jun, Myc, and early growth response gene 1 (Egr-1) transcription factors is significantly extended by sustained extracellular signal-regulated kinase 1 and 2 (ERK1 and -2) signaling. Several of these proteins contain functional docking site for ERK, FXFP (DEF) domains that serve to locally concentrate the active kinase, thus showing that they can function as ERK sensors. Sustained ERK signaling regulates the posttranslational modifications of these IEG-encoded sensors, which contributes to their sustained expression during the G(1)-S transition. DEF domain-containing sensors can also interpret the small changes in ERK signal strength that arise from less than a threefold reduction in agonist concentration. As a result, downstream target gene expression and cell cycle progression are significantly changed.
Figures





References
-
- Angel, P., and M. Karin. 1991. The role of Jun, Fos and the AP-1 complex in cell-proliferation and transformation. Biochim. Biophys. Acta 1072:129-157. - PubMed
-
- Balmanno, K., and S. J. Cook. 1999. Sustained MAP kinase activation is required for the expression of cyclin D1, p21Cip1 and a subset of AP-1 proteins in CCL39 cells. Oncogene 18:3085-3097. - PubMed
-
- Bebien, M., S. Salinas, C. Becamel, V. Richard, L. Linares, and R. A. Hipskind. 2003. Immediate-early gene induction by the stresses anisomycin and arsenite in human osteosarcoma cells involves MAPK cascade signaling to Elk-1, CREB and SRF. Oncogene 22:1836-1847. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
