Assessment of splice variant-specific functions of desmocollin 1 in the skin
- PMID: 14673151
- PMCID: PMC303333
- DOI: 10.1128/MCB.24.1.154-163.2004
Assessment of splice variant-specific functions of desmocollin 1 in the skin
Abstract
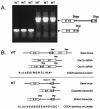
Desmocollin 1 (Dsc1) is part of a desmosomal cell adhesion receptor formed in terminally differentiating keratinocytes of stratified epithelia. The dsc1 gene encodes two proteins (Dsc1a and Dsc1b) that differ only with respect to their COOH-terminal cytoplasmic amino acid sequences. On the basis of in vitro experiments, it is thought that the Dsc1a variant is essential for assembly of the desmosomal plaque, a structure that connects desmosomes to the intermediate filament cytoskeleton of epithelial cells. We have generated mice that synthesize a truncated Dsc1 receptor that lacks both the Dsc1a- and Dsc1b-specific COOH-terminal domains. This mutant transmembrane receptor, which does not bind the common desmosomal plaque proteins plakoglobin and plakophilin 1, is integrated into functional desmosomes. Interestingly, our mutant mice did not show the epidermal fragility previously observed in dsc1-null mice. This suggests that neither the Dsc1a- nor the Dsc1b-specific COOH-terminal cytoplasmic domain is required for establishing and maintaining desmosomal adhesion. However, a comparison of our mutants with dsc1-null mice suggests that the Dsc1 extracellular domain is necessary to maintain structural integrity of the skin.
Figures








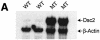
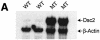
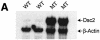
References
-
- Amagai, M., N. Matsuyoshi, Z. H. Wang, C. Andl, and J. R. Stanley. 2000. Toxin in bullous impetigo and staphylococcal scalded-skin syndrome targets desmoglein 1. Nat. Med. 6:1275-1277. - PubMed
-
- Arnemann, J., K. H. Sullivan, A. I. Magee, I. A. King, and R. S. Buxton. 1993. Stratification-related expression of isoforms of the desmosomal cadherins in human epidermis. J. Cell Sci. 104(Pt. 3):741-750. - PubMed
-
- Barker, N., and H. Clevers. 2000. Catenins, Wnt signaling and cancer. Bioessays 22:961-965. - PubMed
-
- Bierkamp, C., K. J. McLaughlin, H. Schwarz, O. Huber, and R. Kemler. 1996. Embryonic heart and skin defects in mice lacking plakoglobin. Dev. Biol. 180:780-785. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
