CD3/CD28 costimulation-induced NF-kappaB activation is mediated by recruitment of protein kinase C-theta, Bcl10, and IkappaB kinase beta to the immunological synapse through CARMA1
- PMID: 14673152
- PMCID: PMC303359
- DOI: 10.1128/MCB.24.1.164-171.2003
CD3/CD28 costimulation-induced NF-kappaB activation is mediated by recruitment of protein kinase C-theta, Bcl10, and IkappaB kinase beta to the immunological synapse through CARMA1
Abstract
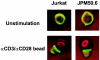
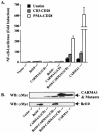
CARMA1 (also known as CARD11) is a scaffold molecule and contains a caspase-recruitment domain (CARD) and a membrane-associated guanylate kinase-like (MAGUK) domain. It plays an essential role in mediating CD3/CD28 costimulation-induced NF-kappaB activation. However, the molecular mechanism by which CARMA1 mediates costimulatory signals remains to be determined. Here, we show that CARMA1 is constitutively associated with the cytoplasmic membrane. This membrane association is essential for the function of CARMA1, since a mutant of CARMA1, CARMA1(L808P), that is defective in the membrane association cannot rescue CD3/CD28 costimulation-induced NF-kappaB activation in JPM50.6 CARMA1-deficient T cells. Although CD3/CD28 costimulation effectively induces the formation of the immunological synapse in CARMA1-deficient T cells, the recruitment of protein kinase C-theta (PKC-theta), Bcl10, and IkappaB kinase beta (IKKbeta) into lipid rafts of the immunological synapse is defective. Moreover, expression of wild-type CARMA1, but not CARMA1(L808P), restores the recruitment of PKC-theta, Bcl10, and IKKbeta into lipid rafts in CARMA1-deficient T cells. Consistently, expression of a mutant CARMA1, CARMA1(DeltaCD), that cannot associate with Bcl10 failed to restore CD3/CD28 costimulation-induced NF-kappaB activation in JPM50.6 cells, whereas expression of Bcl10-CARMA(DeltaCD) fusion protein effectively restored this NF-kappaB activation. Together, these results indicate that CARMA1 mediates CD3/CD28 costimulation-induced NF-kappaB activation by recruiting downstream signaling components into the immunological synapse.
Figures




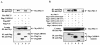


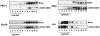
References
-
- Baldwin, A. J. 1996. The NF-κB and IκB proteins: new discoveries and insights. Annu. Rev. Immunol. 14:649-683. - PubMed
-
- Bertin, J., L. Wang, Y. Guo, M. D. Jacobson, J. L. Poyet, S. M. Srinivasula, S. Merriam, P. S. DiStefano, and E. S. Alnemri. 2001. CARD11 and CARD14 are novel caspase recruitment domain (CARD)/membrane-associated guanylate kinase (MAGUK) family members that interact with BCL10 and activate NF-κB. J. Biol. Chem. 276:11877-11882. - PubMed
-
- Bi, K., Y. Tanaka, N. Coudronniere, K. Sugie, S. Hong, M. J. van Stipdonk, and A. Altman. 2001. Antigen-induced translocation of PKC-θ to membrane rafts is required for T cell activation. Nat. Immunol. 2:556-563. - PubMed
-
- Bromley, S. K., W. R. Burack, K. G. Johnson, K. Somersalo, T. N. Sims, C. Sumen, M. M. Davis, A. S. Shaw, P. M. Allen, and M. L. Dustin. 2001. The immunological synapse. Annu. Rev. Immunol. 19:375-396. - PubMed
-
- Clements, J. L., N. J. Boerth, J. R. Lee, and G. A. Koretzky. 1999. Integration of T cell receptor-dependent signaling pathways by adapter proteins. Annu. Rev. Immunol. 17:89-108. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
