Cannabinoid-induced presynaptic inhibition at the primary afferent trigeminal synapse of juvenile rat brainstem slices
- PMID: 14673184
- PMCID: PMC1664814
- DOI: 10.1113/jphysiol.2003.056986
Cannabinoid-induced presynaptic inhibition at the primary afferent trigeminal synapse of juvenile rat brainstem slices
Abstract
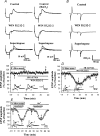
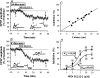
Systemic or intraventricular administration of cannabinoids causes analgesic effects, but relatively little is known for their cellular mechanism. Using brainstem slices with the mandibular nerve attached, we examined the effect of cannabinoids on glutamatergic transmission in superficial trigeminal caudal nucleus of juvenile rats. The exogenous cannabinoid receptor agonist WIN 55,212-2 (WIN), as well as the endogenous agonist anandamide, hyperpolarized trigeminal caudal neurones and depressed the amplitude of excitatory postsynaptic potentials (EPSPs) or currents (EPSCs) monosynaptically evoked by stimulating mandibular nerves in a concentration-dependent manner. The inhibitory action of WIN was blocked or fully reversed by the CB1 receptor antagonist SR 141716A. WIN had no effect on the amplitude of miniature excitatory postsynaptic currents (mEPSCs) recorded in the presence of tetrodotoxin or cadmium. The inhibitory effect of WIN on EPSCs was greater for those with longer synaptic latency, suggesting that cannabinoids have a stronger effect on C-fibre EPSPs than on Adelta-fibre EPSPs. Ba2+ (100 microm) blocked the hyperpolarizing effect of cannabinoids, but did not affect their inhibitory effect on EPSPs. The N-type Ca2+ channel blocker omega-conotoxin GVIA (omega-CgTX) occluded the WIN-mediated presynaptic inhibition, whereas the P/Q-type Ca2+ channel blocker omega-agatoxin TK (omega-Aga) had no effect. These results suggest that cannabinoids preferentially activate CB1 receptors at the nerve terminal of small-diameter primary afferent fibres. Upon activation, CB1 receptors may selectively inhibit presynaptic N-type Ca2+ channels and exocytotic release machinery, thereby attenuating the transmitter release at the trigeminal nociceptive synapses.
Figures



References
-
- Ameri A. The effects of cannabinoids on the brain. Prog Neurobiol. 1999;58:315–348. - PubMed
-
- Ataka T, Kumamoto E, Shimoji K, Yoshimura M. Baclofen inhibits more effectively C-afferent than Aδ-afferent glutamatergic transmission in substantia gelatinosa neurons of adult rat spinal cord slices. Pain. 2000;86:273–282. - PubMed
-
- Auclair N, Otani S, Soubrie P, Crépel F. Cannabinoids modulate synaptic strength and plasticity at glutamatergic synapses of rat prefrontal cortex pyramidal neurons. J Neurophysiol. 2000;83:3287–3293. - PubMed
-
- Basbaum AI, Jessell TM. The perception of pain. In: Kandel ER, Schwartz JH, Jessell TM, editors. Principles of Neural Science. 4. Columbus: McGraw-Hill Companies; 2000. pp. 472–491.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

