Early stage Parkinson's disease patients and normal volunteers: comparative mechanisms of sequence learning
- PMID: 14673808
- PMCID: PMC6871797
- DOI: 10.1002/hbm.10142
Early stage Parkinson's disease patients and normal volunteers: comparative mechanisms of sequence learning
Abstract
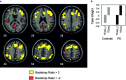
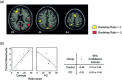
Early-stage nondemented Parkinson's disease (PD(es)) patients can learn short but not long sequences as well as controls. We have previously shown that to achieve normal performance, PD(es) patients activated the same right-sided cortical regions as controls plus the homologous left sided cortex and bilateral cerebellum. In this study, we evaluated two related hypotheses to explain the behavioral abnormalities and the increased bilateral brain activation observed in the PD(es) group. Hypothesis 1 proposed that PD(es) patients recruit regions from a normal bilateral network specialized for sequence learning that healthy controls would activate if performing difficult tasks. Thus, PD(es) patients can learn short sequences as well as controls. Hypothesis 2 proposed that information processing within the network in the PD(es) group is impaired. Thus, PD(es) patients cannot learn as difficult a sequence as controls. To test hypothesis 1, we increased task difficulty and statistical power in the control group and showed that the control and the PD(es) groups activated the same regions. To test hypothesis 2, we analyzed the equal performance data using two partial least squares (PLS) multivariate analyses. The task-PLS analysis showed that to perform equally with controls, the PD(es) group expressed the normal bilateral network more than the control group. The behavior-PLS analysis showed that the correlation between learning performance and regional activation was significantly different between the groups. We conclude that PD(es) patients have near normal learning if task difficulty is moderate because they can recruit additional regions from a normal bilateral network specialized for sequence learning. However, when a difficult task would normally require bilateral activation, PD(es) patients fail to learn because information processing within the network is impaired. Hum. Brain Mapp. 20:246-258, 2003.
Copyright 2003 Wiley-Liss, Inc.
Figures

Similar articles
-
Functional networks in motor sequence learning: abnormal topographies in Parkinson's disease.Hum Brain Mapp. 2001 Jan;12(1):42-60. doi: 10.1002/1097-0193(200101)12:1<42::aid-hbm40>3.0.co;2-d. Hum Brain Mapp. 2001. PMID: 11198104 Free PMC article.
-
Enhancement of brain activation during trial-and-error sequence learning in early PD.Neurology. 2003 Feb 25;60(4):612-9. doi: 10.1212/01.wnl.0000044154.92143.dc. Neurology. 2003. PMID: 12601101 Clinical Trial.
-
Reduced neural connectivity but increased task-related activity during working memory in de novo Parkinson patients.Hum Brain Mapp. 2015 Apr;36(4):1554-66. doi: 10.1002/hbm.22723. Epub 2015 Jan 17. Hum Brain Mapp. 2015. PMID: 25598397 Free PMC article.
-
Functional imaging of sequence learning in Parkinson's disease.J Neurol Sci. 2006 Oct 25;248(1-2):72-7. doi: 10.1016/j.jns.2006.05.005. Epub 2006 Jun 5. J Neurol Sci. 2006. PMID: 16753182 Clinical Trial.
-
Functional brain networks in Parkinson's disease.Parkinsonism Relat Disord. 2001 Oct;8(2):91-4. doi: 10.1016/s1353-8020(01)00022-0. Parkinsonism Relat Disord. 2001. PMID: 11489673 Review.
Cited by
-
Disentangling motor planning and motor execution in unmedicated de novo Parkinson's disease patients: An fMRI study.Neuroimage Clin. 2019;22:101784. doi: 10.1016/j.nicl.2019.101784. Epub 2019 Mar 19. Neuroimage Clin. 2019. PMID: 30925383 Free PMC article.
-
Abnormal structure-function relationships in hereditary dystonia.Neuroscience. 2009 Nov 24;164(1):220-9. doi: 10.1016/j.neuroscience.2008.12.041. Epub 2009 Jan 1. Neuroscience. 2009. PMID: 19162138 Free PMC article. Review.
-
Effects of task prioritization on a postural-motor task in early-stage Parkinson's disease: EEG connectivity and clinical implication.Geroscience. 2022 Aug;44(4):2061-2075. doi: 10.1007/s11357-022-00516-4. Epub 2022 Jan 17. Geroscience. 2022. PMID: 35039998 Free PMC article.
-
Motor Memory Consolidation Deficits in Parkinson's Disease: A Systematic Review with Meta-Analysis.J Parkinsons Dis. 2023;13(6):865-892. doi: 10.3233/JPD-230038. J Parkinsons Dis. 2023. PMID: 37458048 Free PMC article.
-
Striatal and cerebellar interactions during reward-based motor performance.bioRxiv [Preprint]. 2025 Feb 10:2025.02.06.636434. doi: 10.1101/2025.02.06.636434. bioRxiv. 2025. Update in: Proc Natl Acad Sci U S A. 2025 Aug 12;122(32):e2503373122. doi: 10.1073/pnas.2503373122. PMID: 39975096 Free PMC article. Updated. Preprint.
References
-
- Boecker H, Dagher A, Ceballos‐Baumann AO, Passingham RE, Samuel M, Friston KJ, Poline J, Dettmers C, Conrad B, Brooks DJ (1998): Role of the human rostral supplementary motor area and the basal ganglia in motor sequence control: investigations with H2 15O PET. J Neurophysiol 792: 1070–1080. - PubMed
-
- Brown RG (1999): The role of cortico‐striatal circuits in learning sequential information In Stern GM, editor. Parkinson's disease: advances in neurology Philadelphia: Lippincott Williams & Wilkins; 80: 31–39. - PubMed
-
- Brown RG, Marsden CD (1990): Cognitive function in Parkinson's disease: from description to theory. Trends Neurosci 131: 21–29. - PubMed
-
- Catalan MJ, Ishii K, Honda M, Samii A, Hallett M (1999): A PET study of sequential finger movements of varying length in patients with Parkinson's disease. Brain 1223: 483–495. - PubMed
-
- Dhawan V, Kazumata K, Robeson W, Belakhlef A, Margouleff C, Chaly T, Nakamura T, Dahl R, Margouleff D, Eidelberg D (1998): Quantitative brain PET: Comparison of 2D and 3‐D acquisition on the GE Advance Scanner. Clin Posit Imag 12: 135–144. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

