Conversion of peripheral CD4+CD25- naive T cells to CD4+CD25+ regulatory T cells by TGF-beta induction of transcription factor Foxp3
- PMID: 14676299
- PMCID: PMC2194145
- DOI: 10.1084/jem.20030152
Conversion of peripheral CD4+CD25- naive T cells to CD4+CD25+ regulatory T cells by TGF-beta induction of transcription factor Foxp3
Abstract
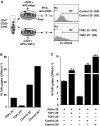
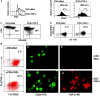
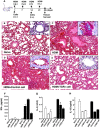
CD4+CD25+ regulatory T cells (Treg) are instrumental in the maintenance of immunological tolerance. One critical question is whether Treg can only be generated in the thymus or can differentiate from peripheral CD4+CD25- naive T cells. In this paper, we present novel evidence that conversion of naive peripheral CD4+CD25- T cells into anergic/suppressor cells that are CD25+, CD45RB-/low and intracellular CTLA-4+ can be achieved through costimulation with T cell receptors (TCRs) and transforming growth factor beta (TGF-beta). Although transcription factor Foxp3 has been shown recently to be associated with the development of Treg, the physiological inducers for Foxp3 gene expression remain a mystery. TGF-beta induced Foxp3 gene expression in TCR-challenged CD4+CD25- naive T cells, which mediated their transition toward a regulatory T cell phenotype with potent immunosuppressive potential. These converted anergic/suppressor cells are not only unresponsive to TCR stimulation and produce neither T helper cell 1 nor T helper cell 2 cytokines but they also express TGF-beta and inhibit normal T cell proliferation in vitro. More importantly, in an ovalbumin peptide TCR transgenic adoptive transfer model, TGF-beta-converted transgenic CD4+CD25+ suppressor cells proliferated in response to immunization and inhibited antigen-specific naive CD4+ T cell expansion in vivo. Finally, in a murine asthma model, coadministration of these TGF-beta-induced suppressor T cells prevented house dust mite-induced allergic pathogenesis in lungs.
Figures



References
-
- Sakaguchi, S. 2000. Regulatory T cells: key controllers of immunologic self-tolerance. Cell. 101:455–458. - PubMed
-
- Shevach, E.M. 2002. CD4+ CD25+ suppressor T cells: more questions than answers. Nat. Rev. Immunol. 2:389–400. - PubMed
-
- Itoh, M., T. Takahashi, N. Sakaguchi, Y. Kuniyasu, J. Shimizu, F. Otsuka, and S. Sakaguchi. 1999. Thymus and autoimmunity: production of CD25+CD4+ naturally anergic and suppressive T cells as a key function of the thymus in maintaining immunologic self-tolerance. J. Immunol. 162:5317–5326. - PubMed
-
- Apostolou, I., A. Sarukhan, L. Klein, and H. von Boehmer. 2002. Origin of regulatory T cells with known specificity for antigen. Nat. Immunol. 3:756–763. - PubMed
-
- Zhang, X., L. Izikson, L. Liu, and H.L. Weiner. 2001. Activation of CD25(+)CD4(+) regulatory T cells by oral antigen administration. J. Immunol. 167:4245–4253. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

