A tenascin-C aptamer identified by tumor cell SELEX: systematic evolution of ligands by exponential enrichment
- PMID: 14676325
- PMCID: PMC307582
- DOI: 10.1073/pnas.2136683100
A tenascin-C aptamer identified by tumor cell SELEX: systematic evolution of ligands by exponential enrichment
Abstract
The targeting of molecular repertoires to complex systems rather than biochemically pure entities is an accessible approach that can identify proteins of biological interest. We have probed antigens presented by a monolayer of tumor cells for their ability to interact with a pool of aptamers. A glioblastoma-derived cell line, U251, was used as the target for systematic evolution of ligands by exponential enrichment by using a single-stranded DNA library. We isolated specifically interacting oligonucleotides, and biochemical strategies were used to identify the protein target for one of the aptamers. Here we characterize the interaction of the DNA aptamer, GBI-10, with tenascin-C, an extracellular protein found in the tumor matrix. Tenascin-C is believed to be involved in both embryogenesis and oncogenesis pathways. Systematic evolution of ligands by exponential enrichment appears to be a successful strategy for the a priori identification of targets of biological interest within complex systems.
Figures
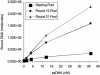

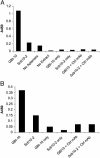
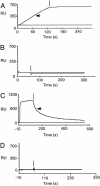
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

