Effect of age on the pharmacokinetics of duloxetine in women
- PMID: 14678340
- PMCID: PMC1884424
- DOI: 10.1046/j.1365-2125.2003.01963.x
Effect of age on the pharmacokinetics of duloxetine in women
Abstract
Aims: The effect of age on duloxetine pharmacokinetics was evaluated in healthy volunteers and in patients with urinary incontinence.
Methods: Twenty-four healthy subjects (12 women 65-77 years, and 12 women 32-50 years) were given a single 40-mg oral dose of duloxetine in Study 1. Plasma concentration-time data were analysed by noncompartmental pharmacokinetic methods. Sparse plasma samples were obtained from patients with urinary incontinence treated in two phase II studies: 70 women (24-77 years) who received duloxetine 20 mg day(-1), 30 mg day(-1), or 40 mg day(-1) in Study 2A and 128 women (28-64 years) who received duloxetine 20 mg day(-1), 40 mg day(-1), or 80 mg day(-1) in Study 2B. Based upon the combined data, a model was developed to characterize population pharmacokinetics of duloxetine using the nonlinear mixed-effects modelling program (NONMEM).
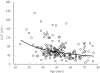
Results: In Study 1, the elderly (> or = 65 years) exhibited a statistically significant slower elimination rate constant lambdaz compared with younger subjects [elderly-younger difference = -0.022 h(-1)[95% confidence interval (CI) -0.036, -0.008]]. However, no statistically significant differences in either CL/F [elderly-younger difference = -17.4 l h(-1) (95% CI -41.1, 6.23)] or V/F [elderly-younger difference = 115.9 l (95% CI -168.6, 400.4)] were observed. The population pharmacokinetic analysis of Studies 2A and 2B revealed that the CL/F of duloxetine decreased with increasing age. Despite statistical significance, the age effect only accounted for 3% of the interindividual variability in CL/F and unexplained sources of the variation in clearance were still substantial (> 50%). Adverse events were generally mild to moderate, and the incidence of adverse events was generally similar in elderly and non-elderly participants in these studies.
Conclusions: Whereas the results suggest that age has an effect on duloxetine pharmacokinetics, primarily reflected as a slower lambdaz in the elderly, the magnitude of mean changes in CL/F, or V/F was small relative to the large interindividual variation in pharmacokinetics. Elderly participants had a safety profile of duloxetine comparable to their younger counterparts. Specific dose recommendations for duloxetine in the elderly are not warranted.
Figures
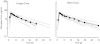
 ) and mean (•) plasma concentration-time profiles of duloxetine obtained from elderly and younger subjects administered a single oral 40-mg dose of duloxetine.
) and mean (•) plasma concentration-time profiles of duloxetine obtained from elderly and younger subjects administered a single oral 40-mg dose of duloxetine.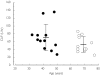
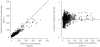
References
-
- Wong DT, Bymaster FP, Mayle DA, Reid LR, Krushinski JH, Robertson DW. LY248686, a new inhibitor of serotonin and norepinephrine uptake. Neuropsychopharmacology. 1993;8:23–33. - PubMed
-
- Thor KB, Katofiasc MA. Effects of duloxetine, a combined serotonin and norepinephrine reuptake inhibitor, on central neural control of lower urinary tract function in the chloralose-anesthetized female cat. J Pharmacol Exp Ther. 1995;274:1014–1024. - PubMed
-
- Katoh A, Eigyo M, Ishibashi C, et al. Behavioral and electroencephalographic properties of duloxetine (LY248686), a reuptake inhibitor of norepinephrine and serotonin, in mice and rats. J Pharmacol Exp Ther. 1995;272:1067–1075. - PubMed
-
- Sharma A, Goldberg MJ, Cerimele BJ. Pharmacokinetics and safety of duloxetine, a dual-serotonin and norepinephrine reuptake inhibitor. J Clin Pharmacol. 2000;40:161–167. - PubMed
-
- Roberts J, Tumer N. Pharmacodynamic basis for altered drug action in the elderly. Clin Geriatr Med. 1988;4:127–149. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

