Functional expression of TASK-1/TASK-3 heteromers in cerebellar granule cells
- PMID: 14678492
- PMCID: PMC1664745
- DOI: 10.1113/jphysiol.2003.054387
Functional expression of TASK-1/TASK-3 heteromers in cerebellar granule cells
Abstract
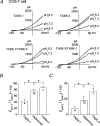
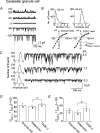
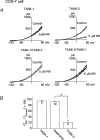
TASK-1 and TASK-3 are functional members of the tandem-pore K+ (K2P) channel family, and mRNAs for both channels are expressed together in many brain regions. Although TASK-1 and TASK-3 subunits are able to form heteromers when their complementary RNAs are injected into oocytes, whether functional heteromers are present in the native tissue is not known. Using cultured cerebellar granule (CG) neurones that express mRNAs of both TASK-1 and TASK-3, we studied the presence of heteromers by comparing the sensitivities of cloned and native K+ channels to extracellular pH (pHo) and ruthenium red. The single-channel conductance of TASK-1, TASK-3 and a tandem construct (TASK-1/TASK-3) expressed in COS-7 cells were 14.2 +/- 0.4, 37.8 +/- 0.7 and 38.1 +/- 0.7 pS (-60 mV), respectively. TASK-3 and TASK-1/TASK-3 (and TASK-3/TASK-1) displayed nearly identical single-channel kinetics. TASK-3 and TASK-1/TASK-3 expressed in COS-7 cells were inhibited by 26 +/- 4 and 36 +/- 2 %, respectively, when pHo was changed from 8.3 to 7.3. In outside-out patches from CG neurones, the K+ channel with single channel properties similar to those of TASK-3 was inhibited by 31 +/- 7 % by the same reduction in pHo. TASK-3 and TASK-1/TASK-3 expressed in COS-7 cells were inhibited by 78 +/- 7 and 3 +/- 4 %, respectively, when 5 microm ruthenium red was applied to outside-out patches. In outside-out patches from CG neurones containing a 38 pS channel, two types of responses to ruthenium red were observed. Ruthenium red inhibited the channel activity by 77 +/- 5 % in 42 % of patches (range: 72-82 %) and by 5 +/- 4 % (range: 0-9 %) in 58 % of patches. When patches contained more than three 38 pS channels, the average response to ruthenium red was 47 +/- 6 % inhibition (n= 5). These electrophysiological studies show that native 38 pS K+ channels of the TASK family in cultured CG neurones consist of both homomeric TASK-3 and heteromeric TASK-1/TASK-3.
Figures




Similar articles
-
Identification of native rat cerebellar granule cell currents due to background K channel KCNK5 (TASK-2).Brain Res Mol Brain Res. 2004 Sep 28;128(2):112-20. doi: 10.1016/j.molbrainres.2004.06.007. Brain Res Mol Brain Res. 2004. PMID: 15363886
-
Characterization of four types of background potassium channels in rat cerebellar granule neurons.J Physiol. 2002 Jul 15;542(Pt 2):431-44. doi: 10.1113/jphysiol.2002.017590. J Physiol. 2002. PMID: 12122143 Free PMC article.
-
Single-channel properties and pH sensitivity of two-pore domain K+ channels of the TALK family.Biochem Biophys Res Commun. 2004 Mar 19;315(4):836-44. doi: 10.1016/j.bbrc.2004.01.137. Biochem Biophys Res Commun. 2004. PMID: 14985088
-
What are the roles of the many different types of potassium channel expressed in cerebellar granule cells?Cerebellum. 2003;2(1):11-25. doi: 10.1080/14734220310015593. Cerebellum. 2003. PMID: 12882230 Review.
-
Pharmacology of neuronal background potassium channels.Neuropharmacology. 2003 Jan;44(1):1-7. doi: 10.1016/s0028-3908(02)00339-8. Neuropharmacology. 2003. PMID: 12559116 Review.
Cited by
-
Structural Insights into the Mechanisms and Pharmacology of K2P Potassium Channels.J Mol Biol. 2021 Aug 20;433(17):166995. doi: 10.1016/j.jmb.2021.166995. Epub 2021 Apr 20. J Mol Biol. 2021. PMID: 33887333 Free PMC article. Review.
-
Nerve growth factor-induced endocytosis of TWIK-related acid-sensitive K⁺ 1 channels in adrenal medullary cells and PC12 cells.Pflugers Arch. 2013 Jul;465(7):1051-64. doi: 10.1007/s00424-013-1222-3. Epub 2013 Feb 2. Pflugers Arch. 2013. PMID: 23377568
-
K(+) channels in O(2) sensing and postnatal development of carotid body glomus cell response to hypoxia.Respir Physiol Neurobiol. 2013 Jan 1;185(1):44-56. doi: 10.1016/j.resp.2012.07.005. Epub 2012 Jul 16. Respir Physiol Neurobiol. 2013. PMID: 22801091 Free PMC article. Review.
-
Mutation of a single residue promotes gating of vertebrate and invertebrate two-pore domain potassium channels.Nat Commun. 2019 Feb 15;10(1):787. doi: 10.1038/s41467-019-08710-3. Nat Commun. 2019. PMID: 30770809 Free PMC article.
-
The Impact of Heterozygous KCNK3 Mutations Associated With Pulmonary Arterial Hypertension on Channel Function and Pharmacological Recovery.J Am Heart Assoc. 2017 Sep 9;6(9):e006465. doi: 10.1161/JAHA.117.006465. J Am Heart Assoc. 2017. PMID: 28889099 Free PMC article.
References
-
- Barhanin J, Lesage F, Guillemare E, Fink M, Lazdunski M, Romey G. K(V)LQT1 and lsK (minK) proteins associate to form the I(Ks) cardiac potassium current. Nature. 1996;384:78–80. - PubMed
-
- Brickley SG, Revilla V, Cull-Candy SG, Wisden W, Farrant M. Adaptive regulation of neuronal excitability by a voltage-independent potassium conductance. Nature. 2001;409:88–92. - PubMed
-
- Chesler M. The regulation and modulation of pH in the nervous system. Prog Neurobiol. 1990;34:401–427. - PubMed
-
- Czirjak G, Enyedi P. Formation of functional heterodimers between the TASK-1 and TASK-3 two-pore domain potassium channel subunits. J Biol Chem. 2002a;277:5426–5432. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

