Pacemaker channels in mouse thalamocortical neurones are regulated by distinct pathways of cAMP synthesis
- PMID: 14678496
- PMCID: PMC1664735
- DOI: 10.1113/jphysiol.2003.050989
Pacemaker channels in mouse thalamocortical neurones are regulated by distinct pathways of cAMP synthesis
Abstract
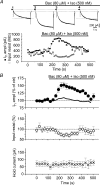
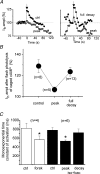
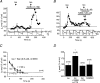
A crucial aspect of pacemaker current (Ih) function is the regulation by cyclic nucleotides. To assess the endogenous mechanisms controlling cAMP levels in the vicinity of pacemaker channels, Ih regulation by G-protein-coupled neurotransmitter receptors was studied in mouse thalamocortical neurones. Activation of beta-adrenergic receptors with (-)-isoproterenol (Iso) led to a small steady enhancement of Ih amplitude, whereas activation of GABAB receptors with (+/-)-Baclofen (Bac) reduced Ih, consistent with an up- and down-regulation of basal cAMP levels, respectively. In contrast, a transient (taudecay, approximately 200 s), supralinear up-regulation of Ih was observed upon coapplication of Iso and Bac that was larger than that observed with Iso alone. This up-regulation appeared to involve a cAMP synthesis pathway distinct from that recruited by Iso, as it was associated with a reversible acceleration in Ih activation kinetics and an occlusion of modulation by photolytically released cAMP, yet showed an 11 mV as opposed to a 6 mV positive shift in the activation curve and an at least seven-fold increase in duration. GABA, in the presence of the GABAA antagonist picrotoxin, mimicked, whereas N-ethylmaleimide, an inhibitor of Gi-proteins, blocked the up-regulation, supporting a requirement for GABAB receptor activation in the potentiation. Activation of synaptic GABAB responses via stimulation of inhibitory afferents from the nucleus reticularis potentiated Iso-induced increments in Ih, suggesting that synaptically located receptors couple positively to cAMP synthesis induced by beta-adrenergic receptors. These findings indicate that distinct pathways of cAMP synthesis target the pacemaker current and the recruitment of these may be controlled by GABAergic activity within thalamic networks.
Figures




References
-
- Andrade R. Enhancement of β-adrenergic responses by Gi-linked receptors in rat hippocampus. Neuron. 1993;10:83–88. - PubMed
-
- Anholt RRH. Signal integration in the nervous system: adenylate cyclases as molecular coincidence detectors. Trends Neurosci. 1994;17:37–41. - PubMed
-
- Bacskai BJ, Hochner B, Mahaut-Smith M, Adams SR, Kaang BK, Kandel ER, Tsien RY. Spatially resolved dynamics of cAMP and protein kinase A subunits in Aplysia sensory neurons. Science. 1993;260:222–226. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

