Gonadotropin releasing hormone analogue (GnRHa) alters the expression and activation of Smad in human endometrial epithelial and stromal cells
- PMID: 14678567
- PMCID: PMC317376
- DOI: 10.1186/1477-7827-1-125
Gonadotropin releasing hormone analogue (GnRHa) alters the expression and activation of Smad in human endometrial epithelial and stromal cells
Retraction in
-
Retraction Note to: Gonadotropin releasing hormone analogue (GnRHa) alters the expression and activation of Smad in human endometrial epithelial and stromal cells.Reprod Biol Endocrinol. 2015 Apr 3;13:25. doi: 10.1186/s12958-015-0016-1. Reprod Biol Endocrinol. 2015. PMID: 25879939 Free PMC article. No abstract available.
Abstract
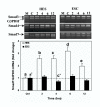
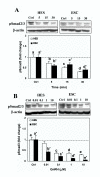
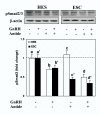
Gonadotropin releasing hormone analogues (GnRHa) are often used to regress endometriosis implants and prevent premature luteinizing hormone surges in women undergoing controlled ovarian stimulation. In addition to GnRH central action, the expression of GnRH and receptors in the endometrium implies an autocrine/paracrine role for GnRH and an additional site of action for GnRHa. To further examine the direct action of GnRH (Leuprolide acetate) in the endometrium, we determined the effect of GnRH on endometrial stromal (ESC) and endometrial surface epithelial (HES) cells expression and activation of Smads (Smad3, -4 and -7), intracellular signals activated by transforming growth factor beta (TGF-beta), a key cytokine expressed in the endometrium. The results show that GnRH (0.1 microM) increased the expression of inhibitory Smad7 mRNA in HES with a limited effect on ESC, while moderately increasing the common Smad4 and Smad7 protein levels in these cells (P < 0.05). GnRH in a dose--(0.01 to 10 microM) and time--(5 to 30 min) dependent manner decreased the rate of Smad3 activation (phospho-Smad3, pSmad3), and altered Smad3 cellular distribution in both cell types. Pretreatment with Antide (GnRH antagonist) resulted in further suppression of Smad3 induced by GnRH, with Antide inhibition of pSmad3 in ESC. Furthermore, co-treatment of the cells with GnRH + TGF-beta, or pretreatment with TGF-beta type II receptor antisense to block TGF-beta autocrine/paracrine action, in part inhibited TGF-beta activated Smad3. In conclusion, the results indicate that GnRH acts directly on the endometrial cells altering the expression and activation of Smads, a mechanism that could lead to interruption of TGF-beta receptor signaling mediated through this pathway in the endometrium.
Figures




Comment in
-
Findings of Research Misconduct.Fed Regist. 2017 Aug 3;82(148):36150-36151. Fed Regist. 2017. PMID: 28857091 Free PMC article. No abstract available.
References
-
- Takeuchi H, Kobori H, Kikuchi I, Sato Y, Mitsuhashi N. A prospective randomized study comparing endocrinological and clinical effects of two types of GnRH agonists in cases of uterine leiomyomas or endometriosis. J Obstet Gynaecol Res. 2000;26:325–331. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

