Auricular chondritis in NOD.DQ8.Abetao (Ag7-/-) transgenic mice resembles human relapsing polychondritis
- PMID: 14679179
- PMCID: PMC296991
- DOI: 10.1172/JCI17450
Auricular chondritis in NOD.DQ8.Abetao (Ag7-/-) transgenic mice resembles human relapsing polychondritis
Abstract
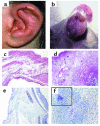
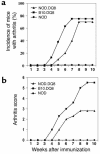
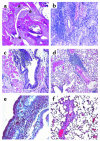
Relapsing polychondritis is a multisystem autoimmune disease involving cartilage destruction but no known causative antigen. HLA-DQ8 has been associated with various autoimmune diseases in humans. To study the role of DQ8 in autoimmune diseases, we have generated transgenic mice expressing DQ8 (DQA1*0301, DQB1*0302) in a NOD background lacking endogenous class II molecules (Abetao). Upon immunization with type II collagen (CII), 85% of NOD.DQ8 mice develop severe experimental polychondritis, auricular chondritis, and polyarthritis, with clinical and histological similarities to relapsing polychondritis (RP) in humans. CII-immunized mice mount a T cell response and produce Ab's to type IX collagen (CIX) and self-CII. Transgene-negative littermates do not develop any serological and clinical manifestations following immunization. B10.DQ8 transgenic mice develop polyarthritis and Ab's to CII only. The susceptibility to auricular chondritis in NOD.DQ8 mice can be attributed to response to CIX. A higher number of activated cells, CD4+CD44(hi)CD62L(lo), and lower regulatory cells CD4+CD152+CD25+ were observed in NOD.DQ8 mice compared with B10.DQ8 mice. The NOD.DQ8 mice provide a model of RP with a high disease incidence and multiple organ involvement to investigate putative autoantigen and regulatory cells involved in disease pathogenesis. An experimental model restricted by the human class II molecule will be valuable when studying the role of various collagens in immunologic and pathologic responses in human RP.
Figures

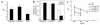

References
-
- Zeuner M, Straub RH, Albert ED, Scholmerich J, Lang B. Relapsing polychondritis: clinical and immunogenetic analysis of 62 patients. J. Rheumatol. 1997;24:96–101. - PubMed
-
- McAdam LP, O’Hanlan MA, Bluestone R, Pearson CM. Relapsing polychondritis: perspective study of 23 patients and a review of literature. Medicine. 1976;55:193–215. - PubMed
-
- Issak BL, Liesegang TJ, Michet CJ. Ocular and systemic findings in relapsing polychondritis. Opthalmology. 1986;93:681–689. - PubMed
-
- Homma S, Matsumoto T, Abe H, Fukuda Y, Suzuki M. Relapsing polychondritis. Pathological and immunological findings in an autopsy case. Acta Pathol. Jpn. 1984;34:1137–1146. - PubMed
-
- Damiani JM, Levine HL. Relapsing polychondritis: report of ten cases. Laryngoscope. 1979;89:929–946. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

