IFN-regulatory factor 3-dependent gene expression is defective in Tbk1-deficient mouse embryonic fibroblasts
- PMID: 14679297
- PMCID: PMC314168
- DOI: 10.1073/pnas.2237236100
IFN-regulatory factor 3-dependent gene expression is defective in Tbk1-deficient mouse embryonic fibroblasts
Abstract
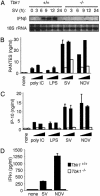
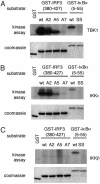
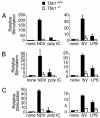
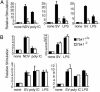
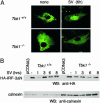
Virus infection, double-stranded RNA, and lipopolysaccharide each induce the expression of genes encoding IFN-alpha and -beta and chemokines, such as RANTES (regulated on activation, normal T cell expressed and secreted) and IP-10 (IFN-gamma inducible protein 10). This induction requires the coordinate activation of several transcription factors, including IFN-regulatory factor 3 (IRF3). The signaling pathways leading to IRF3 activation are triggered by the binding of pathogen-specific products to Toll-like receptors and culminate in the phosphorylation of specific serine residues in the C terminus of IRF3. Recent studies of human cell lines in culture have implicated two noncanonical IkappaB kinase (IKK)-related kinases, IKK-epsilon and Traf family member-associated NF-kappaB activator (TANK)-binding kinase 1 (TBK1), in the phosphorylation of IRF3. Here, we show that purified recombinant IKK-epsilon and TBK1 directly phosphorylate the critical serine residues in IRF3. We have also examined the expression of IRF3-dependent genes in mouse embryonic fibroblasts (MEFs) derived from Tbk1(-/-) mice, and we show that TBK1 is required for the activation and nuclear translocation of IRF3 in these cells. Moreover, Tbk1(-/-) MEFs show marked defects in IFN-alpha and -beta, IP-10, and RANTES gene expression after infection with either Sendai or Newcastle disease viruses or after engagement of the Toll-like receptors 3 and 4 by double-stranded RNA and lipopolysaccharide, respectively. Finally, TRIF (TIR domain-containing adapter-inducing IFN-beta), fails to activate IRF3-dependent genes in Tbk1(-/-) MEFs. We conclude that TBK1 is essential for IRF3-dependent antiviral gene expression.
Figures

Comment in
-
Unexpected similarities in cellular responses to bacterial and viral invasion.Proc Natl Acad Sci U S A. 2004 Jan 20;101(3):695-6. doi: 10.1073/pnas.0307303101. Epub 2004 Jan 12. Proc Natl Acad Sci U S A. 2004. PMID: 14718669 Free PMC article. No abstract available.
References
-
- Janeway, C. A., Jr., & Medzhitov, R. (2002) Annu. Rev. Immunol. 20, 197-216. - PubMed
-
- Medzhitov, R., Preston-Hurlburt, P. & Janeway, C. A., Jr. (1997) Nature 388, 394-397. - PubMed
-
- Akira, S. (2001) Adv. Immunol. 78, 1-56. - PubMed
-
- Dunne, A. & O'Neill, L. A. (2003) Sci. STKE 2003, re3. - PubMed
-
- Maniatis, T. (1986) Harvey Lect. 82, 71-104. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

