Electrical stimulation directly induces pre-angiogenic responses in vascular endothelial cells by signaling through VEGF receptors
- PMID: 14679307
- PMCID: PMC1459284
- DOI: 10.1242/jcs.00868
Electrical stimulation directly induces pre-angiogenic responses in vascular endothelial cells by signaling through VEGF receptors
Abstract
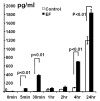
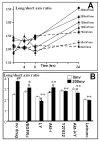
Controlling angiogenesis is crucial. Growth factors and cytokines are key regulators but a full understanding remains elusive. Endogenous electrical potential differences exist within and around the vasculature, both in relation to blood flow and in situations where active angiogenesis occurs, such as wound healing, development and tumor growth. Recent work shows that electrical stimulation induces significant angiogenesis in vivo, through enhanced vascular endothelial growth factor (VEGF) production by muscle cells. We report that applied electric fields (EFs) of small physiological magnitude directly stimulate VEGF production by endothelial cells in culture without the presence of any other cell type. EFs as low as 75-100 mV mm-1 (1.5-2.0 mV across an endothelial cell) directed the reorientation, elongation and migration of endothelial cells in culture. These pre-angiogenic responses required VEGF receptor activation and were mediated through PI3K-Akt and Rho-ROCK signaling pathways, resulting in reorganization of the actin cytoskeleton. This indicates that endogenous EFs might play a role in angiogenesis in vivo by stimulating the VEGF receptor signaling pathway, to induce key pre-angiogenic responses. In addition, it raises the feasibility of using applied EFs to initiate and guide angiogenesis through direct effects on endothelial cells.
Figures




References
-
- Albuquerque ML, Waters CM, Savla U, Schnaper HW, Flozak AS. Shear stress enhances human endothelial cell wound closure in vitro. Am J Physiol Heart Circ Physiol. 2000;279:H293–H302. - PubMed
-
- Barker AT, Jaffe LF, Vanable JW., Jr The glabrous epidermis of cavies contains a powerful battery. Am J Physiol. 1982;242:R358–R366. - PubMed
-
- Borgens RB, Shi R. Uncoupling histogenesis from morphogenesis in the vertebrate embryo by collapse of the transneural tube potential. Dev Dyn. 1995;203:456–467. - PubMed
-
- Brent TP, Forrester JA. Changes in surface charge of HeLa cells during the cell cycle. Nature. 1967;215:92–93. - PubMed
-
- Burger PC, Chandler DB, Klintworth GK. Corneal neovascularization as studied by scanning electron microscopy of vascular casts. Lab Invest. 1983;48:169–180. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

