Localized in vivo 13C NMR spectroscopy of the brain
- PMID: 14679498
- PMCID: PMC1513184
- DOI: 10.1002/nbm.841
Localized in vivo 13C NMR spectroscopy of the brain
Abstract
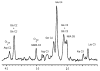
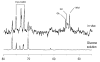
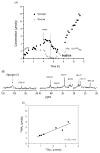
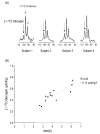
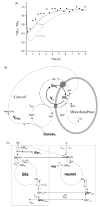
Localized (13)C NMR spectroscopy provides a new investigative tool for studying cerebral metabolism. The application of (13)C NMR spectroscopy to living intact humans and animals presents the investigator with a number of unique challenges. This review provides in the first part a tutorial insight into the ingredients required for achieving a successful implementation of localized (13)C NMR spectroscopy. The difficulties in establishing (13)C NMR are the need for decoupling of the one-bond (13)C-(1)H heteronuclear J coupling, the large chemical shift range, the low sensitivity and the need for localization of the signals. The methodological consequences of these technical problems are discussed, particularly with respect to (a) RF front-end considerations, (b) localization methods, (c) the low sensitivity, and (d) quantification methods. Lastly, some achievements of in vivo localized (13)C NMR spectroscopy of the brain are reviewed, such as: (a) the measurement of brain glutamine synthesis and the feasibility of quantifying glutamatergic action in the brain; (b) the demonstration of significant anaplerotic fluxes in the brain; (c) the demonstration of a highly regulated malate-aspartate shuttle in brain energy metabolism and isotope flux; (d) quantification of neuronal and glial energy metabolism; and (e) brain glycogen metabolism in hypoglycemia in rats and humans. We conclude that the unique and novel insights provided by (13)C NMR spectroscopy have opened many new research areas that are likely to improve the understanding of brain carbohydrate metabolism in health and disease.
Copyright 2003 John Wiley & Sons, Ltd.
Figures









References
-
- Chance EM, Seeholzer SH, Kobayashi K, Williamson JR. Mathematical analysis of isotope labeling in the citric acid cycle with applications to 13C NMR studies in perfused rat hearts. . J Biol Chem. 1983;258:13785–13794. - PubMed
-
- Malloy C, Sherry A, Jeffrey F. Analysis of tricarboxylic acid cycle of the heart using 13C isotope isomers. . Am J Physiol. 1990;259:H987–H995. - PubMed
-
- Weiss RG, Stern MD, de Albuquerque CP, Vandegaer K, Chacko VP, Gerstenblith G. Consequences of altered aspartate amino-transferase activity on 13C-glutamate labelling by the tricarboxylic acid cycle in intact rat hearts. . Biochim Biophys Acta. 1995;1243:543–548. - PubMed
-
- Yu X, Alpert NM, Lewandowski ED. Modeling enrichment kinetics from dynamic 13C NMR spectra: theoretical analysis and practical considerations. . Am J Physiol. 1997;41:C2037–C2048. - PubMed
-
- van Zijl PCM, Rothman D. NMR studies of brain C-13-glucose uptake and metabolism—present status. . Magn Reson Imag. 1995;13:1213–1221. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

