Both cyclin B levels and DNA-replication checkpoint control the early embryonic mitoses in Drosophila
- PMID: 14681192
- PMCID: PMC3825093
- DOI: 10.1242/dev.00944
Both cyclin B levels and DNA-replication checkpoint control the early embryonic mitoses in Drosophila
Abstract
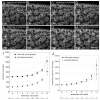
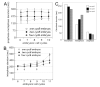
The earliest embryonic mitoses in Drosophila, as in other animals except mammals, are viewed as synchronous and of equal duration. However, we observed that total cell-cycle length steadily increases after cycle 7, solely owing to the extension of interphase. Between cycle 7 and cycle 10, this extension is DNA-replication checkpoint independent, but correlates with the onset of Cyclin B oscillation. In addition, nuclei in the middle of embryos have longer metaphase and shorter anaphase than nuclei at the two polar regions. Interestingly, sister chromatids move faster in anaphase in the middle than the posterior region. These regional differences correlate with local differences in Cyclin B concentration. After cycle 10, interphase and total cycle duration of nuclei in the middle of the embryo are longer than at the poles. Because interphase also extends in checkpoint mutant (grapes) embryo after cycle 10, although less dramatic than wild-type embryos, interphase extension after cycle 10 is probably controlled by both Cyclin B limitation and the DNA-replication checkpoint.
Figures




References
-
- Blangy A, Lane HA, d’Herin P, Harper M, Kress M, Nigg EA. Phosphorylation by p34cdc2 regulates spindle association of human Eg5, a kinesin-related motor essential for bipolar spindle formation in vivo. Cell. 1995;83:1159–1169. - PubMed
-
- Blankenship JT, Wieschaus E. Two new roles for the Drosophila AP patterning system in early morphogenesis. Development. 2001;128:5129–5138. - PubMed
-
- Clarkson M, Saint R. A His2AvDGFP fusion gene complements a lethal His2AvD mutant allele and provides an in vivo marker for Drosophila chromosome behavior. DNA Cell Biol. 1999;18:457–462. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

