Selective abolition of pancreatic RNase binding to its inhibitor protein
- PMID: 14681553
- PMCID: PMC314137
- DOI: 10.1073/pnas.0307268101
Selective abolition of pancreatic RNase binding to its inhibitor protein
Abstract
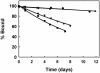
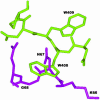
We have modified RNase inhibitor (RI) protein so that it no longer detectably binds pancreatic RNases but retains near-native affinity for human angiogenin (ANG). The K(i) value for RNase A is increased by a factor of >10(8), from 36 fM to >4 microM, and the selectivity factor for ANG is now >10(9). This dramatic change was achieved by remodeling the human RI loop segment Cys-408 -Leu-409 -Gly-410, which makes minor interactions with pancreatic RNase but does not contact ANG. The modifications selected were designed to sterically hinder docking of the undesired ligand. Three of the variants tested (C408W, G410W, and C408W/G410W) bind RNase A with almost the same avidity as WT RI. However, combination of the 408/410 double Trp replacement with deletion of the intervening residue, Leu-409, was sufficient to abolish inhibition of RNase A and human pancreatic RNase. The K(i) value for ANG with the deletion variant is 1.1 fM, only 2-fold higher than with WT RI. This variant may have potential utility both as an anticancer drug targeting ANG and as a tool for the investigation of the biological function of ANG. More generally, these findings demonstrate that a protein-protein interaction can be effectively and specifically disrupted by redesigning an interface region that makes no major energetic contribution to complex stability. This finding, in turn, may have implications for the development of small molecules that modulate protein-protein interactions.
Figures


References
-
- Skowyra, D., Craig, K. L., Tyers, M., Elledge, S. J. & Harper, J. W. (1997) Cell 91, 209-219. - PubMed
-
- Orlicky, S., Tang, X. J., Willems, A., Tyers, M. & Sicheri, F. (2003) Cell 112, 243-256. - PubMed
-
- Lupher, M. L., Rao, N., Eck, M. J. & Band, H. (1999) Immunol. Today 20, 375-382. - PubMed
-
- Wells, J. A. & de Vos, A. M. (1996) Annu. Rev. Biochem. 65, 609-634. - PubMed
-
- Jeffrey, P. D., Russo, A. A., Polyak, K., Gibbs, E., Hurwitz, J., Massague, J. & Pavletich, N. P. (1995) Nature 376, 313-320. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

