T cell chemotaxis to lysophosphatidylcholine through the G2A receptor
- PMID: 14681556
- PMCID: PMC314170
- DOI: 10.1073/pnas.2536801100
T cell chemotaxis to lysophosphatidylcholine through the G2A receptor
Abstract
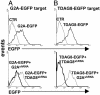
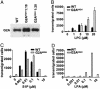
G2A is an immunoregulatory G protein-coupled receptor predominantly expressed in lymphocytes and macrophages. Ectopic overexpression studies have implicated G2A as a receptor for the bioactive lysophospholipid, lysophosphatidylcholine (LPC). However, the functional consequences of LPC-G2A interaction at physiological levels of receptor expression, and in a cellular context relevant to its immunological role, remain largely unknown. Here, we show impaired chemotaxis to LPC of a T lymphoid cell line in which G2A expression was chronically down-regulated by RNA interference technology. Rescuing this phenotype by reconstitution of the physiological level of receptor expression further supports a functional connection between LPC-G2A interaction and cellular motility. Overexpression of G2A in the T lymphoid cell line significantly enhanced chemotaxis to LPC. It also modified migration toward the LPC-related molecule, lysophosphatidic acid, indicating the possibility of crosstalk between G2A and endogenous lysophosphatidic acid receptors. The role of G2A in LPC-mediated cell migration may be relevant to the autoimmune syndrome associated with genetic inactivation of this G protein-coupled receptor in mice. The experimental system described here can be useful for understanding the structural requirements for LPC recognition by G2A and the signaling pathways regulated by this ligand-receptor pair.
Figures



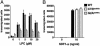

References
-
- Lombardi, M. S., Kavelaars, A. & Heijnen, C. J. (2002) Crit. Rev. Immunol. 22, 141-163. - PubMed
-
- Fukushima, N., Ishii, I., Contos, J. J., Weiner, J. A. & Chun, J. (2001) Annu. Rev. Pharmacol. Toxicol. 41, 507-534. - PubMed
-
- Kabarowski, J. H., Xu, Y. & Witte, O. N. (2002) Biochem. Pharmacol. 64, 161-167. - PubMed
-
- Kabarowski, J. H. S., Zhu, K., Le, L. Q., Witte, O. N. & Xu, Y. (2001) Science 293, 702-705. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

